Androgens protect against apolipoprotein E4-induced cognitive deficits - PubMed (original) (raw)
Androgens protect against apolipoprotein E4-induced cognitive deficits
Jacob Raber et al. J Neurosci. 2002.
Abstract
Compared with apolipoprotein (apo) E2 and E3, apoE4 increases the risk of Alzheimer's disease (AD), but it remains unknown how apoE4 affects neuronal function. ApoE4 interacts with female gender, further increasing the risk of AD and decreasing treatment response. Female mice are also more susceptible to apoE4-induced impairments of spatial learning and memory than male mice. To assess the role of sex steroids in this process, we studied mice deficient in mouse apoE (Apoe(-/-)) and expressing human apoE4 or apoE3 in the brain at comparable levels. Even brief periods of androgen treatment improved the memory deficits of female apoE4 mice. Female apoE3 mice had no memory deficits and did not benefit from the treatment. ApoE4 male mice, which performed normally in a water-maze test at baseline, developed prominent deficits in spatial learning and memory after blockade of androgen receptors (ARs), whereas apoE3 male mice did not. Untreated apoE4 mice had significantly lower cytosolic AR levels in the neocortex than wild-type, Apoe(-/-), and apoE3 mice. Improved memory in androgen-treated female apoE4 mice was associated with increased cytosolic AR levels. Our findings suggest that apoE4 contributes to cognitive decline by reducing AR levels in the brain, and that stimulating AR-dependent pathways can reverse apoE4-induced cognitive deficits.
Figures
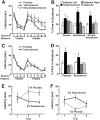
Fig. 1.
ApoE4-induced deficits in spatial learning and memory can be improved by stimulating ARs. Six-month-old female NSE–apoE4 and NSE–apoE3 mice were tested in the water maze beginning 8 d after the subcutaneous implantation of testosterone, dihydrotestosterone, or placebo capsules (n = 5–11 mice per genotype and treatment). After the behavioral testing, plasma hormone levels were determined by radioimmunoassay to ensure the effectiveness of the implants (see Results). A, Testosterone improved spatial learning in female apoE4 mice. Although all three groups learned to locate the hidden platform, there was a significant difference between the learning curves of testosterone- and placebo-treated mice during the hidden-platform sessions (p < 0.05; repeated-measures ANOVA).B, In the probe trial (platform removed), both testosterone- and dihydrotestosterone-treated apoE4 mice, but not placebo-treated apoE4 mice, spent significantly more time searching in the target quadrant than in any of the other quadrants (*p < 0.05; Tukey–Kramer test). However, the dihydrotestosterone effect was relatively weak. Although the ratio of mice spending ≥35% versus <35% in the target quadrant was higher after testosterone (5:0) than after placebo (1:4) treatment (p < 0.01; χ2 test), it did not differ significantly from placebo after dihydrotestosterone treatment (5:4; p = 0.2; χ2 test). Learning curves calculated from distance moved were similar to those calculated from latencies (data not shown). C, D, Testosterone did not improve spatial learning and memory in female apoE3 mice. *p < 0.05 versus any other quadrant (Tukey–Kramer test). E, F, Trials 1–3 of the first hidden-platform session. Latency values for each session (A, C) represent the averages from three consecutive trials. Comparison of latencies in each of the first three hidden-platform trials by ANOVA revealed a significant interaction between treatment and genotype during trials 2 (p = 0.002) and 3 (p = 0.027) but not trial 1 (p = 0.95). Comparison of the four learning curves by repeated-measures ANOVA also identified a significant interaction between treatment and genotype (p = 0.028). Moreover, the effect of genotype was significant only in the placebo-treated groups (p = 0.002) but not in the testosterone-treated groups (p = 0.93).
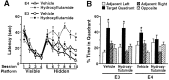
Fig. 2.
Blockade of ARs impaired spatial learning and memory in male apoE4 but not apoE3 mice. Six-month-old male NSE–apoE4 and NSE–apoE3 mice (n = 6–7 mice per genotype and treatment) were tested in the water maze during AR blockade with hydroxyflutamide (see Materials and Methods). A, Hydroxyflutamide impaired learning in the hidden-platform sessions in apoE4 mice (p < 0.05 vs vehicle-treated apoE4 mice; repeated-measures ANOVA) but not in apoE3 mice.B, Hydroxyflutamide impaired retention of spatial memory in apoE4 but not apoE3 mice. *p < 0.05 versus any other quadrant (Tukey–Kramer test).
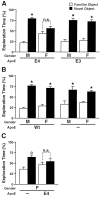
Fig. 3.
ApoE4 impairs object-recognition memory in female mice. Novel-object recognition was tested in untreated 6-month-old male (M) and female (F) mice. The results shown are from the memory-retention session.A, NSE–apoE4 (11 males, 7 females) and NSE–apoE3 (4 males, 7 females) mice. B, Wild-type (Wt) (11 males, 6 females) and Apoe −/− (7 males, 13 females) mice. C,Apoe−/− (6 females) and GFAP–apoE4 (6 females) mice. Most mice showed normal object-recognition memory. Only female mice that expressed apoE4 in neurons (A) or astrocytes (C) failed to spend significantly more time with the novel than with the familiar object. °p < 0.05; *p < 0.01 versus time exploring the familiar object (Tukey–Kramer test); n.s., not significant.
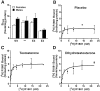
Fig. 4.
Cytosolic AR levels in the neocortex and response to androgen treatment. Total cytosolic AR levels were determined from AR saturation curves using a single curve-fit analysis. Results are expressed as femtomoles of [3H]R1881 bound per milligram of protein. There were no differences in_K_d among the groups (data not shown).A, Untreated male and female NSE–apoE4 mice had lower cytosolic AR levels than untreated NSE–apoE3,Apoe −/−, and wild-type (Wt) mice (n = 3–7 mice per gender and genotype). B–D, Effect of placebo (B), testosterone (C), and dihydrotestosterone (D) on AR saturation curves in female NSE–apoE4 mice (n = 3–6 mice per treatment).
Similar articles
- Role of circulating androgen levels in effects of apoE4 on cognitive function.
Pfankuch T, Rizk A, Olsen R, Poage C, Raber J. Pfankuch T, et al. Brain Res. 2005 Aug 16;1053(1-2):88-96. doi: 10.1016/j.brainres.2005.06.028. Brain Res. 2005. PMID: 16054121 - Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior.
Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Grootendorst J, et al. Behav Brain Res. 2005 Apr 15;159(1):1-14. doi: 10.1016/j.bbr.2004.09.019. Epub 2004 Nov 6. Behav Brain Res. 2005. PMID: 15794991 - Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks.
Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Bour A, et al. Behav Brain Res. 2008 Nov 21;193(2):174-82. doi: 10.1016/j.bbr.2008.05.008. Epub 2008 May 18. Behav Brain Res. 2008. PMID: 18572260 - Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.
Baum L, Chen L, Ng HK, Pang CP. Baum L, et al. Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review. - Androgens, apoE, and Alzheimer's disease.
Raber J. Raber J. Sci Aging Knowledge Environ. 2004 Mar 17;2004(11):re2. doi: 10.1126/sageke.2004.11.re2. Sci Aging Knowledge Environ. 2004. PMID: 15028864 Review.
Cited by
- Early effects of whole-body (56)Fe irradiation on hippocampal function in C57BL/6J mice.
Haley GE, Yeiser L, Olsen RH, Davis MJ, Johnson LA, Raber J. Haley GE, et al. Radiat Res. 2013 May;179(5):590-6. doi: 10.1667/RR2946.1. Epub 2013 Mar 19. Radiat Res. 2013. PMID: 23510274 Free PMC article. - Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice.
Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y. Leung L, et al. PLoS One. 2012;7(12):e53569. doi: 10.1371/journal.pone.0053569. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300939 Free PMC article. - ApoE and cerebral insulin: Trafficking, receptors, and resistance.
Rhea EM, Raber J, Banks WA. Rhea EM, et al. Neurobiol Dis. 2020 Apr;137:104755. doi: 10.1016/j.nbd.2020.104755. Epub 2020 Jan 21. Neurobiol Dis. 2020. PMID: 31978603 Free PMC article. Review. - Regional-specific effects of ovarian hormone loss on synaptic plasticity in adult human APOE targeted replacement mice.
Klein RC, Saini S, Risher ML, Acheson SK, Fleming RL, Sexton HG, Swartzwelder HS, Moore SD. Klein RC, et al. PLoS One. 2014 Apr 14;9(4):e94071. doi: 10.1371/journal.pone.0094071. eCollection 2014. PLoS One. 2014. PMID: 24732142 Free PMC article. - Simulated spatial radiation impacts learning and memory ability with alterations of neuromorphology and gut microbiota in mice.
Song C, Gao X, Song W, Zeng D, Shan S, Yin Y, Li Y, Baranenko D, Lu W. Song C, et al. RSC Adv. 2020 Apr 23;10(27):16196-16208. doi: 10.1039/d0ra01017k. eCollection 2020 Apr 21. RSC Adv. 2020. PMID: 35493686 Free PMC article.
References
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull. 1997;43:279–287. - PubMed
- Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc. 1999;47:1289–1293. - PubMed
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm Behav. 1979;12:112–163. - PubMed
- Benson S. Hormone replacement therapy and Alzheimer's disease: an update on the issues. Health Care Women Int. 1999;20:619–638. - PubMed
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous