A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status - PubMed (original) (raw)
A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status
James G Rheinwald et al. Mol Cell Biol. 2002 Jul.
Abstract
With increasing frequency during serial passage in culture, primary human keratinocytes express p16(INK4A) (p16) and undergo senescence arrest. Keratinocytes engineered to express hTERT maintain long telomeres but typically are not immortalized unless, by mutation or other heritable event, they avoid or greatly reduce p16 expression. We have confirmed that keratinocytes undergo p16-related senescence during growth in culture, whether in the fibroblast feeder cell system or in the specialized K-sfm medium formulation, and that this mechanism can act as a barrier to immortalization following hTERT expression. We have characterized the p16-related arrest mechanism more precisely by interfering specifically with several regulators of cell cycle control. Epidermal, oral mucosal, corneal limbal, and conjunctival keratinocytes were transduced to express a p16-insensitive mutant cdk4 (cdk4(R24C)), to abolish p16 control, and/or a dominant negative mutant p53 (p53DD), to abolish p53 function. Expression of either cdk4(R24C) or p53DD alone had little effect on life span, but expression of both permitted cells to divide 25 to 43 population doublings (PD) beyond their normal limit. Keratinocytes from a p16(+/-) individual transduced to express p53DD alone displayed a 31-PD life span extension associated with selective growth of variants that had lost the wild-type p16 allele. Cells in which both p53 and p16 were nonfunctional divided rapidly during their extended life span but experienced telomere erosion and ultimately ceased growth with very short telomeres. Expression of hTERT in these cells immortalized them. Keratinocytes engineered to express cdk4(R24C) and hTERT but not p53DD did not exhibit an extended life span. Rare immortal variants exhibiting p53 pathway defects arose from them, however, indicating that the p53-dependent component of keratinocyte senescence is telomere independent. Mutational loss of p16 and p53 has been found to be a frequent early event in the development of squamous cell carcinoma. Our results suggest that such mutations endow keratinocytes with extended replicative potential which may serve to increase the probability of neoplastic progression.
Figures
FIG. 1.
Cessation of division at the end of life span by primary human keratinocytes in culture is tightly linked to the expression and accumulation of p16INK4A. (a and b) Mid-life-span OKF4 cells serially passaged in K-sfm medium (at ≈25 PD of their ≈37-PD life span). (c and d) Late-life-span OKF4 cells serially passaged in the feeder fibroblast/FAD medium system (at ≈40 PD of their ≈45-PD life span). (e and f) Immortalized OKF4/TERT (early, feeders) cells serially passaged in the feeder fibroblast/FAD medium system (at 70 PD, which is 25 PD beyond the life span limit of the parent cell line). All three cell lines were plated at a low density (≈103 cells/cm2) and allowed to grow for 6 to 8 days before fixation. The fields shown are typical colonies that were the progeny of single cells. (a, c, and e) BUdR had been added to the medium 24 h before fixation, and cells were coimmunostained for p16 (blue) and for BUdR (red/brown). Asterisks in panels d and f indicate large, flat, p16- and BUdR-negative 3T3 fibroblast feeder cells at the periphery of keratinocyte colonies. Note that in the three cell lines shown, nearly all cells were cycling except for those containing moderate to high levels of p16. Small p16-positive cells are rare, indicating that cells become large, flat, and nondividing within several days of expressing p16. (b, d, and f) Phase-contrast images.
FIG. 2.
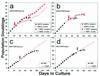
Ability of keratinocytes and fibroblasts to be immortalized by hTERT expression early versus late in their life spans. The primary oral keratinocyte line OKF4 and the primary dermal fibroblast line R2F were transduced with the pBABE(hTERT)puro vector at either an early (a and c) or late (b and d) stage of their respective life spans. Downward arrows indicate the time of transduction. The OKF4 parent line and hTERT transductants were cultured in the fibroblast feeder cell system until drug selection was complete, after which half the cells were subsequently cultured in the feeder system and half in K-sfm medium. ∗ indicates the PD level at which cell populations were completely senescent. Note that R2F fibroblasts were immortalized by hTERT expression at any time during their life span, whereas OKF4 keratinocytes were not immortalized if hTERT was expressed late in the life span in either culture system. OKF4 cells yielded immortalized lines when hTERT was expressed earlier in the life span, but a long period of initially slow population doubling rates preceded the appearance of more rapidly dividing immortalized cells for OKF4/TERT (early) cells cultured in K-sfm, and a modest inflection of the growth curve and progressive selection of more rapidly dividing cells were also evident for OKF4/TERT (early) cells cultured with feeder cells. Small arrowheads at the ends of some lines in the graphs indicate immortalization.
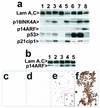
FIG. 3.
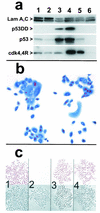
Expression and activity of cdk4R and p53DD in keratinocytes. (a) Western blots detecting expression of p53DD, p53, cdk4, the mutant cdk4R, and nuclear lamins A and C (as loading controls). Lane 1, strain N (34 PD); lane 2, strain N (44 PD); lane 3, N/p53DD (53 PD); lane 4, N/cdk4R/p53DD (69 PD); lane 5, N/cdk4R (46 PD); lane 6, POE9n (40 PD). Note elevated levels of endogenous p53 protein in p53DD-expressing cells and mutant cdk4R protein at severalfold higher levels than endogenous cdk4 protein in cdk4R-expressing cells. (b) cdk4R function in transductants. N/cdk4R cells at 54 PD (near senescence) treated with BUdR 24 h before fixation and stained immunocytochemically for BUdR (red) and p16 (blue). Note S-phase entry (BUdR incorporation) in cells containing high levels of p16. (c) Expression of p53DD in keratinocytes prevents growth arrest in response to DNA damage. Panels 1 and 2, strain N. Panels 3 and 4, N/p53DD. Cells shown in panels 2 and 4 were treated with 0.5 nM actinomycin D for the final 2 days before fixation. Cells shown in all panels were treated with 1 nM BUdR for the final 20 h before fixation. In each panel, the upper square shows BUdR immunocytochemical staining and the lower square is the phase contrast image of the same field. Note absence of normal growth arrest response in N/p53DD cells.
FIG. 4.
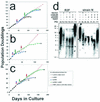
Effect on timing of senescence and susceptibility to hTERT immortalization of inhibiting p53- and p16INK4A-dependent growth arrest mechanisms. Graphs show life span progressions of human primary lines of the following different cell types and transductants thereof: (a) R2F fibroblasts, (b) HuCL-22 keratinocytes, and (c) strain N keratinocytes. Downward arrows indicate the points at which cells were stably transduced with the various vectors. Asterisk (∗) indicates the PD level at which senescence occurred for control cells and for transductants that did not exhibit significant life span extension. + indicates the PD level at which extended life span transductants ceased net population expansion. Small arrowheads at the ends of some lines in the graphs indicate immortalization. (d) Telomere status of normal fibroblasts and keratinocytes and derivatives engineered to bypass their respective senescence mechanisms. Terminal restriction fragment hybridization gels showing terminal restriction fragment lengths (in kilobases on the left) of fibroblast strain R2F and keratinocyte strain N control cells and designated transductants. The PD levels of the cultures analyzed are indicated above each lane.
FIG. 5.
Senescence bypass of p16INK4A+/− keratinocytes engineered to be p53 deficient, displayed by variants that spontaneously lose the wild-type p16INK4A allele. (a) Life span progression of the p16INKA+/− keratinocyte strain K107 and transductants. Symbols are as in Fig. 4. (b) PCR-based single-strand conformational polymorphism analysis detecting wild-type and mutant allele in K107 and transductants thereof at designated PD levels. W, wild-type allele; M, mutant allele; X, unrelated sequence. Note loss of wild-type p16 allele in extended life span K107/p53DD and K107/p53DD/TERT cells but retention of both alleles in K107/p53DD cells transduced to express cdk4R, which presumably acts as a phenocopy of p16 deficiency and removes selection pressure for loss of the wild-type allele. (c) Western blot analysis of p16 expression by K107 and transductants. Lane 1, strain N (40 PD); lane 2, K107 (18 PD); lane 3, K107/p53DD (44 PD); lane 4, K107/p53DD/cdk4R (46 PD); lane 5, POE9n (42 PD). Note the very faint p16 band, presumably of the unstable mutant p16 protein, in lane 3 and the high level of presumably wild-type p16 protein in lane 4. POE9n, which has a homozygous deletion at the p16INK4A locus, served as a negative control. (d to f) p16 immunocytochemical staining. (d) K107 (20 PD). (e) K107/p53DD/cdk4R (39 PD). (f) K107/p53DD (37 PD). Note that extended life span K107/p53DD cells in panel f, which retain only the mutant allele, stained very weakly, presumably because of the short half-life of this mutant p16.
FIG. 6.
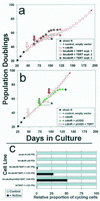
Engineering keratinocytes to resist p16INK4A inhibition and to express hTERT is not sufficient to immortalize them. (a) Strain N cells were first transduced to express cdk4R. Then, in three separate experiments, aliquots of the N/cdk4R population were transduced to express hTERT and serially passaged. Downward arrows indicate timing of the respective transductions. Asterisks indicate complete senescence of the cell population. + indicates evasion of senescence and growth for a substantially extended life span. Note that in all three experiments, the cdk4R/TERT double transductants senesced at the same time or only slightly later than strains N and N/cdk4R. Only experiment 1 yielded a rare immortalized variant. (b) In another experiment, the same N/cdk4R line shown in panel a was first transduced to express p53DD and then to express hTERT. Note that the N/cdk4R/p53DD/TERT cells immediately exhibited an immortalized phenotype, without any inflection in the life span plot that would have suggested outgrowth of a rare variant. (c) Loss of the DNA damage response by rare variants of N/cdk4R/TERT-1 cells that evaded senescence and became immortalized. Cells growing in K-sfm medium were untreated or pretreated with low concentrations of actinomycin D tointroduce DNA strand breaks, received BUdR for the final 20 h before fixation, and were immunostained for BUdR as described in the text. From 150 to 500 cells in at least four randomly selected fields were scored for labeled nuclei. The labeling index (percent cycling cells) of control cultures was 70 to 80% for all lines shown. These control values were normalized to 100% (gray bars), and the relative numbers of labeled nuclei in the actinomycin-treated culture of each line (black bars) are shown in the graph. Note that the parent primary line strain N cells, presenescent N/cdk4R cells, and early N/cdk4R/TERT-1 cells exhibited the normal DNA damage response of cell cycle arrest. In contrast, the later-passage, rapidly dividing, immortalized N/cdk4/TERT-1 cultures and N/cdk4R/p53DD/TERT cultures contained a substantial frequency of cells that were not arrested and continued to cycle following DNA damage. Note also that N/TERT-1, a line previously shown to be deficient in p16 expression but having normal p53 function (17), displayed a normal DNA damage response, demonstrating that p16 deficiency and telomere stabilization alone do not impair this response.
FIG. 7.
Expression of p14INK4A, p21cip1, and p14ARF in normal keratinocytes and cells engineered to bypass senescence by evading p16- and p53-dependent cell cycle controls. (a) Western blot of strain N and derivatives. Lane 1, strain N (26 PD); lane 2, (44 PD); lane 3, strain N (55 PD); lane 4, POE9n (42 PD); lane 5, N/cdk4R (49 PD); lane 6, N/p53DD (55 PD); lane 7, N/cdk4R/p53DD (67 PD); lane 8, N/E6E7 (124 PD). Note that POE9n, which has a homozygous deletion at the p16/p14ARF locus, served as a negative control and N/E6E7 served as a positive control for expression of p16 and p14ARF proteins. (b) Western blot of K107 and derivatives. Lane 1, N/E6E7 (128 PD); lane 2, K107 (20 PD); lane 3, K107/p53DD (44 PD); lane 4, K107/p53DD/cdk4R (40 PD); lane 5, K107/p53DD/TERT (54 PD). (c to f) Immunocytochemical staining of strain N and derivatives for p14ARF (panels c to e) and for p16INK4A (panel f). (c) Strain N (51 PD); (d) N/p53DD/cdk4R (51 PD); (e and f) N/p53DD/cdk4R (58 PD). Inset in panel e is an enlargement showing the characteristic nucleolar location of p14ARF.
FIG. 8.
Differentiation potential of keratinocytes engineered to bypass senescence. (a and b) Epidermal keratinocytes transduced to express cdk4R and p53DD and cultured to within 10 PD of crisis, immunocytochemically stained for K10 (a) and involucrin (b). (c and d) Hematoxylin- and eosin-stained sections of organotypic cultures of the primary epidermal keratinocyte line strain N at 32 PD (c) and of N/cdk4R/p53DD/TERT at 116 PD (d).
Similar articles
- Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics.
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Dickson MA, et al. Mol Cell Biol. 2000 Feb;20(4):1436-47. doi: 10.1128/MCB.20.4.1436-1447.2000. Mol Cell Biol. 2000. PMID: 10648628 Free PMC article. - Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells.
Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW. Ramirez RD, et al. Oncogene. 2003 Jan 23;22(3):433-44. doi: 10.1038/sj.onc.1206046. Oncogene. 2003. PMID: 12545164 - Telomerase activity is sufficient to bypass replicative senescence in human limbal and conjunctival but not corneal keratinocytes.
Pellegrini G, Dellambra E, Paterna P, Golisano O, Traverso CE, Rama P, Lacal P, De Luca M. Pellegrini G, et al. Eur J Cell Biol. 2004 Dec;83(11-12):691-700. doi: 10.1078/0171-9335-00424. Eur J Cell Biol. 2004. PMID: 15679113 - Replicative senescence as a barrier to human cancer.
Parkinson EK, Munro J, Steeghs K, Morrison V, Ireland H, Forsyth N, Fitzsimmons S, Bryce S. Parkinson EK, et al. Biochem Soc Trans. 2000 Feb;28(2):226-33. doi: 10.1042/bst0280226. Biochem Soc Trans. 2000. PMID: 10816133 Review. - Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells.
Yaswen P, Stampfer MR. Yaswen P, et al. Int J Biochem Cell Biol. 2002 Nov;34(11):1382-94. doi: 10.1016/s1357-2725(02)00047-x. Int J Biochem Cell Biol. 2002. PMID: 12200033 Review.
Cited by
- Differential regulation of GLUT1 activity in human corneal limbal epithelial cells and fibroblasts.
Kuipers DP, Scripture JP, Gunnink SM, Salie MJ, Schotanus MP, Ubels JL, Louters LL. Kuipers DP, et al. Biochimie. 2013 Feb;95(2):258-63. doi: 10.1016/j.biochi.2012.09.022. Epub 2012 Sep 23. Biochimie. 2013. PMID: 23009931 Free PMC article. - Differential effects of lovastatin on cisplatin responses in normal human mesothelial cells versus cancer cells: implication for therapy.
Shi Y, Felley-Bosco E, Marti TM, Stahel RA. Shi Y, et al. PLoS One. 2012;7(9):e45354. doi: 10.1371/journal.pone.0045354. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23028957 Free PMC article. - HPV16 E7 protein and hTERT proteins defective for telomere maintenance cooperate to immortalize human keratinocytes.
Miller J, Dakic A, Chen R, Palechor-Ceron N, Dai Y, Kallakury B, Schlegel R, Liu X. Miller J, et al. PLoS Pathog. 2013;9(4):e1003284. doi: 10.1371/journal.ppat.1003284. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592995 Free PMC article. - TERT promoter mutations and methylation for telomerase activation in urothelial carcinomas: New mechanistic insights and clinical significance.
Liu T, Li S, Xia C, Xu D. Liu T, et al. Front Immunol. 2023 Jan 12;13:1071390. doi: 10.3389/fimmu.2022.1071390. eCollection 2022. Front Immunol. 2023. PMID: 36713366 Free PMC article. Review. - Mesoporous Silica-Coated Gold Nanoparticles for Multimodal Imaging and Reactive Oxygen Species Sensing of Stem Cells.
Trayford C, Crosbie D, Rademakers T, van Blitterswijk C, Nuijts R, Ferrari S, Habibovic P, LaPointe V, Dickman M, van Rijt S. Trayford C, et al. ACS Appl Nano Mater. 2022 Mar 25;5(3):3237-3251. doi: 10.1021/acsanm.1c03640. Epub 2022 Mar 14. ACS Appl Nano Mater. 2022. PMID: 35372794 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- K01 CA094223/CA/NCI NIH HHS/United States
- P01 DE012467/DE/NIDCR NIH HHS/United States
- R01 DE013178/DE/NIDCR NIH HHS/United States
- CA 94223/CA/NCI NIH HHS/United States
- R01 DE 13178/DE/NIDCR NIH HHS/United States
- P01 DE 12467/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous