Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells - PubMed (original) (raw)
Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells
Nathaniel N Urban et al. J Physiol. 2002.
Abstract
How patterns of odour-evoked glomerular activity are transformed into patterns of mitral cell action potentials (APs) in the olfactory bulb is determined by the functional connectivity of the cell populations in the bulb. We have used paired whole-cell voltage recordings from olfactory bulb slices to compare the functional connectivity of mitral cells to the known anatomy of the mitral cell network. Both inhibitory and excitatory coupling were observed between pairs of mitral cells. Inhibitory coupling was seen as an increased frequency of small, asynchronous GABAergic IPSPs following APs in the presynaptic cell. Excitatory coupling was short in latency, beginning about 1.3 ms after the presynaptic AP and was mediated by both NMDA and AMPA receptors. Mitral cell pairs were coupled by excitation if and only if their apical dendrites terminated in the same glomerulus. The excitatory coupling between mitral cells resembles conventional fast synaptic transmission in its time course, amplitude and latency, despite the absence of evidence for anatomically defined synapses between mitral cells.
Figures
Figure 1
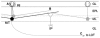
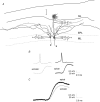
Schematic representation of intra- and interglomerular interactions A single active olfactory bulb mitral or tufted cell (M/T) will relay its activity to other mitral cells associated with the same glomerulus (A), to mitral cells located in other glomeruli up to 2 mm away (B) and also to downstream brain areas via the lateral olfactory tract (LOT; C). Periglomerular cells (pg) are the main local circuit interneurons mediating intraglomerular inhibition whereas granule cells (gc) are the main local circuit interneurons mediating interglomerular inhibition. Dotted circles indicate borders of individual glomeruli. Dendritic lengths in the drawing are approximately to scale, with the circles around the apical tufts having a diameter of approximately 100 μm. GL, glomerular layer; EPL, external plexiform layer; ML, mitral cell layer; GL, granule cell layer.
Figure 2
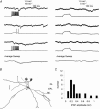
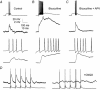
Lateral IPSPs between mitral cells A, left, single pre- and postsynaptic sweeps from a paired recording from two mitral cells show that stimulation of trains of APs in one mitral cell evoked a series of unitary IPSPs in the other. Individual IPSPs were not well correlated with presynaptic APs and the increase in IPSP frequency outlasted the train of presynaptic APs. Right, sweeps from the same pair of cells in which presynaptic depolarization was subthreshold for AP generation show that the spontaneous rate of unitary IPSPs was low and could not account for the IPSPs observed in part A. Bottom traces show the average of 25 consecutive sweeps. B, reconstructed morphology of the pair of mitral cells from which the data in A were obtained. Axons are indicated by ‘a’. Scale bar is 100 μ
m
. C, histogram showing amplitudes of lateral IPSPs evoked by trains of 6–10 presynaptic APs. Average IPSP amplitude was 0.32 mV.
Figure 3
Time course of lateral IPSPs A, lateral IPSPs were evoked in one mitral cell by trains of APs lasting 50–800 ms in a second cell. The time course and amplitude of the lateral IPSP (average of 7 sweeps) were unaffected by the duration of the train of presynaptic APs (single example sweeps shown). B, group data for four cells showing that in experiments similar to those shown in A the latency from the beginning of the presynaptic current injection to the peak of the lateral IPSP was unaffected by changing the duration of the presynaptic current step. _C_1, paired recordings from two mitral cells showing inhibitory coupling. Trains of 5 APs evoked at 20 Hz (top) or 100 Hz (bottom) resulted in IPSPs of similar amplitude and time course in the postsynaptic mitral cell. Postsynaptic traces are averages of 6 sweeps. _C_2, reconstructed morphology of the mitral cell pair from which the data in (_C_1) were recorded. Scale bar is 100 μ
m
.
Figure 5
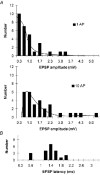
Amplitude and latency histograms of lateral EPSPs A, histograms showing the amplitudes of lateral EPSPs evoked by single presynaptic APs (upper panel) and trains of 10 APs (lower panel) in mitral cell pairs that were found to have apical dendrites terminating in the same glomerulus. Average single AP-evoked EPSP was 0.83 mV and average EPSP evoked by 10 APs was 1.72 mV. B, histogram showing the latency of the lateral EPSPs. Average latency was 1.34 ms.
Figure 4
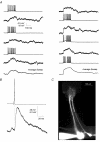
Lateral EPSP between mitral cell pairs A, reciprocal lateral EPSPs were evoked in two mitral cells by trains of APs in the other mitral cell. Left column shows EPSPs evoked in the first cell by the second cell whereas the right column shows EPSPs evoked in second cell by the first. Bottom traces show the average EPSP evoked in the two directions. B, pre- and postsynaptic sweeps from this pair (n = 8) were aligned in time with respect to the peak of the presynaptic AP and averaged. The postsynaptic EPSP shows short latency and fast rise time. Data are from the connection shown in the right-hand column of A. C, fluorescence image of the two cells from which the data above were obtained. Cells were filled with Alexa 548, 20 μ
m
. The image shows that the cells had nearly parallel apical dendrites and apical tufts that terminated in the same glomerulus.
Figure 6
Lateral EPSPs are mediated by synapses on the apical dendrite A, reconstruction of two mitral cells from which the data in B and C were obtained showing the position of the dendritic electrode. Scale bar is 100 μ
m
. B, lateral EPSPs evoked in bicuculline (20 μ
m
) by single APs were recorded in the soma (left traces) and then, after repatching the cell, 180 μ
m
from the soma, in the apical dendrite (right two traces) of the mitral cell. All traces are averages of 15+ sweeps aligned to the peak of the presynaptic AP. C, same postsynaptic traces as in B shown superimposed and at a larger scale to illustrate latency difference.
Figure 8
Lateral EPSPs have a large AMPA receptor-dependent component A, under control conditions presynaptic APs (top) evoked EPSPs in a postsynaptic mitral cell (middle) having its apical tuft in the same glomerulus. Bottom, the same traces are also shown superimposed at an expanded time base. B, addition of bicuculline revealed a large recurrent EPSP in the presynaptic cell (top) and showed that the lateral EPSP observed under control conditions was partially masked by lateral inhibition (middle and bottom). Lateral inhibition reduced the later phases of the lateral EPSP most strongly. C, addition of APV (50 μ
m
) reduced the recurrent EPSP in the presynaptic cell (top) and also reduced the late phases of the lateral EPSP (middle and bottom). The initial peak of the lateral EPSP was, however, unaffected by the APV. D, subsequent to application of bicuculline and APV (left), addition of CNQX (10 μ
m
) blocked the remaining lateral EPSP completely (right).
Figure 7
Mitral cells with co-terminal apical dendrites are often connected by both EPSPs and IPSPs A, reconstruction of two biocytin-filled mitral cells showing that their apical dendritic tufts were located in the same glomerulus. Scale bar is 100 μ
m
. B, pre- and postsynaptic voltage traces from these two cells show that stimulation of APs under control conditions in one cell (left) resulted in an initial depolarization, followed by a longer-lasting hyperpolarization. Right, this hyperpolarization was blocked by addition of bicuculline (20 μ
m
) to the bathing solution.
Similar articles
- Current-source density analysis in the rat olfactory bulb: laminar distribution of kainate/AMPA- and NMDA-receptor-mediated currents.
Aroniadou-Anderjaska V, Ennis M, Shipley MT. Aroniadou-Anderjaska V, et al. J Neurophysiol. 1999 Jan;81(1):15-28. doi: 10.1152/jn.1999.81.1.15. J Neurophysiol. 1999. PMID: 9914263 - Gamma-frequency excitatory input to granule cells facilitates dendrodendritic inhibition in the rat olfactory Bulb.
Halabisky B, Strowbridge BW. Halabisky B, et al. J Neurophysiol. 2003 Aug;90(2):644-54. doi: 10.1152/jn.00212.2003. Epub 2003 Apr 23. J Neurophysiol. 2003. PMID: 12711716 - Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors.
Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Aroniadou-Anderjaska V, et al. J Neurophysiol. 2000 Sep;84(3):1194-203. doi: 10.1152/jn.2000.84.3.1194. J Neurophysiol. 2000. PMID: 10979995 - Electrical signaling in the olfactory bulb.
Lowe G. Lowe G. Curr Opin Neurobiol. 2003 Aug;13(4):476-81. doi: 10.1016/s0959-4388(03)00092-8. Curr Opin Neurobiol. 2003. PMID: 12965296 Review. - Neural encoding of olfactory recognition memory.
Sánchez-Andrade G, James BM, Kendrick KM. Sánchez-Andrade G, et al. J Reprod Dev. 2005 Oct;51(5):547-58. doi: 10.1262/jrd.17031. J Reprod Dev. 2005. PMID: 16284449 Review.
Cited by
- Top-Down Control of Inhibitory Granule Cells in the Main Olfactory Bulb Reshapes Neural Dynamics Giving Rise to a Diversity of Computations.
Chen Z, Padmanabhan K. Chen Z, et al. Front Comput Neurosci. 2020 Jul 13;14:59. doi: 10.3389/fncom.2020.00059. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32765248 Free PMC article. - Intraglomerular inhibition shapes the strength and temporal structure of glomerular output.
Shao Z, Puche AC, Liu S, Shipley MT. Shao Z, et al. J Neurophysiol. 2012 Aug 1;108(3):782-93. doi: 10.1152/jn.00119.2012. Epub 2012 May 16. J Neurophysiol. 2012. PMID: 22592311 Free PMC article. - Granule cell excitability regulates gamma and beta oscillations in a model of the olfactory bulb dendrodendritic microcircuit.
Osinski BL, Kay LM. Osinski BL, et al. J Neurophysiol. 2016 Aug 1;116(2):522-39. doi: 10.1152/jn.00988.2015. Epub 2016 Apr 27. J Neurophysiol. 2016. PMID: 27121582 Free PMC article. - Specific entrainment of mitral cells during gamma oscillation in the rat olfactory bulb.
David FO, Hugues E, Cenier T, Fourcaud-Trocmé N, Buonviso N. David FO, et al. PLoS Comput Biol. 2009 Oct;5(10):e1000551. doi: 10.1371/journal.pcbi.1000551. Epub 2009 Oct 30. PLoS Comput Biol. 2009. PMID: 19876377 Free PMC article. - Stimulus dependent diversity and stereotypy in the output of an olfactory functional unit.
Arneodo EM, Penikis KB, Rabinowitz N, Licata A, Cichy A, Zhang J, Bozza T, Rinberg D. Arneodo EM, et al. Nat Commun. 2018 Apr 9;9(1):1347. doi: 10.1038/s41467-018-03837-1. Nat Commun. 2018. PMID: 29632302 Free PMC article.
References
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Dendrodendritic recurrent excitation in mitral cells of the rat olfactory bulb. Journal of Neurophysiology. 1999;82:489–494. - PubMed
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends in Neurosciences. 1997;20:377–384. - PubMed
- Buonviso N, Chaput MA. Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. Journal of Neurophysiology. 1990;63:447–454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous