Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death - PubMed (original) (raw)
Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death
Sean P Cregan et al. J Cell Biol. 2002.
Abstract
Caspase-independent death mechanisms have been shown to execute apoptosis in many types of neuronal injury. P53 has been identified as a key regulator of neuronal cell death after acute injury such as DNA damage, ischemia, and excitotoxicity. Here, we demonstrate that p53 can induce neuronal cell death via a caspase-mediated process activated by apoptotic activating factor-1 (Apaf1) and via a delayed onset caspase-independent mechanism. In contrast to wild-type cells, Apaf1-deficient neurons exhibit delayed DNA fragmentation and only peripheral chromatin condensation. More importantly, we demonstrate that apoptosis-inducing factor (AIF) is an important factor involved in the regulation of this caspase-independent neuronal cell death. Immunofluorescence studies demonstrate that AIF is released from the mitochondria by a mechanism distinct from that of cytochrome-c in neurons undergoing p53-mediated cell death. The Bcl-2 family regulates this release of AIF and subsequent caspase-independent cell death. In addition, we show that enforced expression of AIF can induce neuronal cell death in a Bax- and caspase-independent manner. Microinjection of neutralizing antibodies against AIF significantly decreased injury-induced neuronal cell death in Apaf1-deficient neurons, indicating its importance in caspase-independent apoptosis. Taken together, our results suggest that AIF may be an important therapeutic target for the treatment of neuronal injury.
Figures
Figure 1.
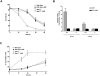
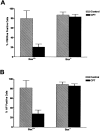
Camptothecin induces neuronal cell death via a caspase- mediated process in the presence of Apaf1 and via a delayed onset caspase-independent pathway in the absence of Apaf1. Wild-type and Apaf1-deficient cortical neurons were treated with camptothecin (10 μM) in the presence or absence of a broad spectrum caspase inhibitor (BAF, 50 μM). (A) Neuronal survival was measured by live/dead assay (n = 4). (B) Caspase-3 activity was determined by DEVD-AFC cleavage (n = 3). (C) The proportion of apoptotic cells was determined by TUNEL staining (n = 3). (D) Nuclear morphology was assessed by Hoechst staining. Bar, 15 μm.
Figure 1.
Camptothecin induces neuronal cell death via a caspase- mediated process in the presence of Apaf1 and via a delayed onset caspase-independent pathway in the absence of Apaf1. Wild-type and Apaf1-deficient cortical neurons were treated with camptothecin (10 μM) in the presence or absence of a broad spectrum caspase inhibitor (BAF, 50 μM). (A) Neuronal survival was measured by live/dead assay (n = 4). (B) Caspase-3 activity was determined by DEVD-AFC cleavage (n = 3). (C) The proportion of apoptotic cells was determined by TUNEL staining (n = 3). (D) Nuclear morphology was assessed by Hoechst staining. Bar, 15 μm.
Figure 2.
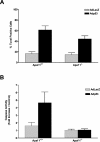
Direct expression of p53 induces neuronal cell death via a caspase-mediated process in the presence of Apaf1 and via a caspase-independent pathway in the absence of Apaf1. Wild-type and Apaf1-deficient neurons were infected with Ad-p53 or the control vector Ad-LacZ at 50 multiplicity of infection (MOI). (A) The fraction of TUNEL-positive cells was measured 72 h after infection (n = 3). (B) Caspase-3 activity was measured at 72 h by DEVD-AFC cleavage (n = 3).
Figure 3.
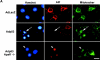
AIF is released from the mitochondria during p53-induced neuronal cell death. (A) Apaf1+/+ and Apaf1−/− cortical neurons were infected at 50 MOI with Ad-p53 or the control vector Ad-LacZ. After 72 h, neurons were labeled with a mitochondrial-specific dye (mitotracker green FM, 0.5 μM), fixed, and immunostained for AIF. The incorporation of mitotracker green FM into mitochondria does not depend on the mitochondrial transmembrane potential. Neurons undergoing p53-mediated cell death exhibit loss of mitochondrial AIF staining (arrows) and nuclear translocation (arrowheads). Bar, 25 μm. (B) Western blot analysis of AIF and cytochrome-c levels in neurons 48 h after infection with Ad-p53 or Ad-LacZ. (C) P53−/−, Apaf1−/−, and corresponding wild-type neurons were treated with camptothecin (10 μM) and then fixed and stained for AIF after 24 h. Representative images were captured and the proportion of cells exhibiting punctate, mitochondrial AIF staining was scored (n = 3).
Figure 3.
AIF is released from the mitochondria during p53-induced neuronal cell death. (A) Apaf1+/+ and Apaf1−/− cortical neurons were infected at 50 MOI with Ad-p53 or the control vector Ad-LacZ. After 72 h, neurons were labeled with a mitochondrial-specific dye (mitotracker green FM, 0.5 μM), fixed, and immunostained for AIF. The incorporation of mitotracker green FM into mitochondria does not depend on the mitochondrial transmembrane potential. Neurons undergoing p53-mediated cell death exhibit loss of mitochondrial AIF staining (arrows) and nuclear translocation (arrowheads). Bar, 25 μm. (B) Western blot analysis of AIF and cytochrome-c levels in neurons 48 h after infection with Ad-p53 or Ad-LacZ. (C) P53−/−, Apaf1−/−, and corresponding wild-type neurons were treated with camptothecin (10 μM) and then fixed and stained for AIF after 24 h. Representative images were captured and the proportion of cells exhibiting punctate, mitochondrial AIF staining was scored (n = 3).
Figure 4.
AIF and cytochrome-c are released by distinct mechanisms during camptothecin-induced neuronal cell death. (A) Apaf1-deficient neurons were treated with camptothecin (10 μM) and at the indicated times, cells were labeled with CMX-Ros to assess mitochondrial transmembrane potential, or fixed and immunostained for AIF or cytochrome-c. Images were captured and the fraction of cells retaining mitochondrial transmembrane potential or exhibiting mitochondrial cytochrome-c/AIF staining was scored (n = 4). (B) Photomicrographs of Apaf1−/− neurons treated with camptothecin for 12 h and stained for AIF, cytochrome-c, or CMX-Ros and the corresponding Hoechst or phase images. Bar, 30 μm.
Figure 4.
AIF and cytochrome-c are released by distinct mechanisms during camptothecin-induced neuronal cell death. (A) Apaf1-deficient neurons were treated with camptothecin (10 μM) and at the indicated times, cells were labeled with CMX-Ros to assess mitochondrial transmembrane potential, or fixed and immunostained for AIF or cytochrome-c. Images were captured and the fraction of cells retaining mitochondrial transmembrane potential or exhibiting mitochondrial cytochrome-c/AIF staining was scored (n = 4). (B) Photomicrographs of Apaf1−/− neurons treated with camptothecin for 12 h and stained for AIF, cytochrome-c, or CMX-Ros and the corresponding Hoechst or phase images. Bar, 30 μm.
Figure 5.
Bax mediates mitochondrial depolarization and the release of AIF during camptothecin-induced neuronal cell death. Wild-type and Bax-deficient neurons were treated with camptothecin and after 24 h, cells were either labeled with CMX-Ros to assess mitochondrial transmembrane potential or fixed and immunostained for AIF. Representative images were taken, and the proportion of cells exhibiting positive mitochondrial staining for CMX-Ros (A) or AIF (B) was scored (n = 3).
Figure 6.
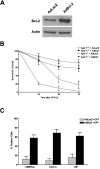
Bcl-2 inhibits camptothecin-induced AIF release and caspase-independent cell death. (A) Western blot analysis of Bcl-2 expression in cortical neurons 48 h after infection with Ad-Bcl-2 or the control vector Ad-LacZ at 50 MOI. (B) Wild-type and Apaf1-deficient cortical neurons were infected with Ad-Bcl-2 or Ad-LacZ for 48 h before treatment with camptothecin (10 μM) or vehicle control, and neuronal survival was determined at the indicated times by live/dead assay (n = 3). (C) Cortical neurons were infected with Ad-Bcl-2 or Ad-LacZ for 48 h and then treated with camptothecin or vehicle control for an additional 48 h. Cells were then labeled with CMX-Ros to assess mitochondrial transmembrane potential, or fixed and immunostained for AIF or cytochrome-c. Representative images were captured, and the fraction of cells maintaining mitochondrial membrane potential or mitochondrial cytochrome-c/AIF staining was scored (n = 3).
Figure 7.
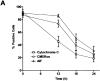
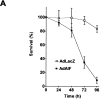
AIF can induce neuronal cell death independent of Apaf1 and caspase activation. Apaf1-deficient neurons were infected at 75 MOI with Ad-AIF or the control vector Ad-LacZ and cultured in the presence or absence of BAF (50 μM). (A) Neuronal survival was determined at the indicated times by MTT assay (n = 4). (B) Photomicrographs of TUNEL staining and nuclear morphology in neurons 72 h after infection with Ad-LacZ or Ad-AIF. Bar, 20 μm. (C) The proportion of apoptotic cells was measured at 72 h by TUNEL staining (n = 3).
Figure 7.
AIF can induce neuronal cell death independent of Apaf1 and caspase activation. Apaf1-deficient neurons were infected at 75 MOI with Ad-AIF or the control vector Ad-LacZ and cultured in the presence or absence of BAF (50 μM). (A) Neuronal survival was determined at the indicated times by MTT assay (n = 4). (B) Photomicrographs of TUNEL staining and nuclear morphology in neurons 72 h after infection with Ad-LacZ or Ad-AIF. Bar, 20 μm. (C) The proportion of apoptotic cells was measured at 72 h by TUNEL staining (n = 3).
Figure 7.
AIF can induce neuronal cell death independent of Apaf1 and caspase activation. Apaf1-deficient neurons were infected at 75 MOI with Ad-AIF or the control vector Ad-LacZ and cultured in the presence or absence of BAF (50 μM). (A) Neuronal survival was determined at the indicated times by MTT assay (n = 4). (B) Photomicrographs of TUNEL staining and nuclear morphology in neurons 72 h after infection with Ad-LacZ or Ad-AIF. Bar, 20 μm. (C) The proportion of apoptotic cells was measured at 72 h by TUNEL staining (n = 3).
Figure 8.
AIF induces neuronal cell death independent of Bax. (A) Wild-type and Bax-deficient neurons were infected with Ad-AIF or Ad-LacZ at increasing MOI, and cell survival was determined at 72 h by MTT assay (n = 3). (B) Neurons were infected with Ad-LacZ or Ad-AIF at 75 MOI and cells were fixed and stained for cytochrome-c and counterstained with Hoechst. Bar, 15 μm.
Figure 8.
AIF induces neuronal cell death independent of Bax. (A) Wild-type and Bax-deficient neurons were infected with Ad-AIF or Ad-LacZ at increasing MOI, and cell survival was determined at 72 h by MTT assay (n = 3). (B) Neurons were infected with Ad-LacZ or Ad-AIF at 75 MOI and cells were fixed and stained for cytochrome-c and counterstained with Hoechst. Bar, 15 μm.
Figure 9.
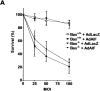
Camptothecin-induced caspase- independent cell death and AIF translocation is inhibited by AIF neutralizing antibodies. (A) Wild-type or (B) Apaf1-deficient neurons were microinjected with either AIF antiserum or preimmune serum along with a fluorescent marker (Alexa®488-dextran) and then treated with camptothecin. After 36 h, cells were fixed, stained with Hoechst, and the fraction of Alexa®-positive cells exhibiting nuclear pyknosis was scored (n = 3). (*, P < 0.01, ANOVA). (C) AIF immunostaining in Apaf1−/− neurons microinjected with preimmune serum or AIF antisera and treated with camptothecin. Bar, 15 μm.
Figure 9.
Camptothecin-induced caspase- independent cell death and AIF translocation is inhibited by AIF neutralizing antibodies. (A) Wild-type or (B) Apaf1-deficient neurons were microinjected with either AIF antiserum or preimmune serum along with a fluorescent marker (Alexa®488-dextran) and then treated with camptothecin. After 36 h, cells were fixed, stained with Hoechst, and the fraction of Alexa®-positive cells exhibiting nuclear pyknosis was scored (n = 3). (*, P < 0.01, ANOVA). (C) AIF immunostaining in Apaf1−/− neurons microinjected with preimmune serum or AIF antisera and treated with camptothecin. Bar, 15 μm.
Similar articles
- Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms.
Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, Park DS, Bennett SA, Slack RS. Cheung EC, et al. J Neurosci. 2005 Feb 9;25(6):1324-34. doi: 10.1523/JNEUROSCI.4261-04.2005. J Neurosci. 2005. PMID: 15703386 Free PMC article. - Two distinct pathways leading to nuclear apoptosis.
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prévost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G. Susin SA, et al. J Exp Med. 2000 Aug 21;192(4):571-80. doi: 10.1084/jem.192.4.571. J Exp Med. 2000. PMID: 10952727 Free PMC article. - p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury.
Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. Seth R, et al. J Biol Chem. 2005 Sep 2;280(35):31230-9. doi: 10.1074/jbc.M503305200. Epub 2005 Jun 27. J Biol Chem. 2005. PMID: 15983031 - Role of mitochondrial proteins for neuronal cell death after focal cerebral ischemia.
Plesnila N. Plesnila N. Acta Neurochir Suppl. 2004;89:15-9. doi: 10.1007/978-3-7091-0603-7_3. Acta Neurochir Suppl. 2004. PMID: 15335097 Review. - Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria.
Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Candé C, et al. Biochimie. 2002 Feb-Mar;84(2-3):215-22. doi: 10.1016/s0300-9084(02)01374-3. Biochimie. 2002. PMID: 12022952 Review.
Cited by
- The DNA-PK catalytic subunit regulates Bax-mediated excitotoxic cell death by Ku70 phosphorylation.
Liu J, Naegele JR, Lin SL. Liu J, et al. Brain Res. 2009 Nov 3;1296:164-75. doi: 10.1016/j.brainres.2009.07.101. Epub 2009 Aug 4. Brain Res. 2009. PMID: 19664609 Free PMC article. - Angiogenin-mediated ribosomal RNA transcription as a molecular target for treatment of head and neck squamous cell carcinoma.
Chen L, Hu GF. Chen L, et al. Oral Oncol. 2010 Sep;46(9):648-53. doi: 10.1016/j.oraloncology.2010.06.011. Epub 2010 Jul 24. Oral Oncol. 2010. PMID: 20656548 Free PMC article. Review. - The molecular mechanisms of cell death in the course of transient ischemia are differentiated in evolutionary distinguished brain structures.
Lietzau G, Kowiański P, Karwacki Z, Dziewiatkowski J, Witkowska M, Sidor-Kaczmarek J, Moryś J. Lietzau G, et al. Metab Brain Dis. 2009 Sep;24(3):507-23. doi: 10.1007/s11011-009-9149-2. Epub 2009 Aug 20. Metab Brain Dis. 2009. PMID: 19693659 - N-terminal deletion augments the cell-death-inducing activity of BAX in adenoviral gene delivery to nonsmall cell lung cancers.
Usui K, Saijo Y, Narumi K, Koyama S, Maemondo M, Kikuchi T, Tazawa R, Hagiwara K, Ishibashi Y, Ohta S, Nukiwa T. Usui K, et al. Oncogene. 2003 May 1;22(17):2655-63. doi: 10.1038/sj.onc.1206331. Oncogene. 2003. PMID: 12730679 - Mitochondrial function in apoptotic neuronal cell death.
Haeberlein SL. Haeberlein SL. Neurochem Res. 2004 Mar;29(3):521-30. doi: 10.1023/b:nere.0000014823.74782.b7. Neurochem Res. 2004. PMID: 15038600 Review.
References
- Adams, J.M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326. - PubMed
- Banasiak, K.J., and G.G. Haddad. 1998. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 797:295–304. - PubMed
- Cecconi, F., G. Alvarez-Bolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. - PubMed
- Cheng, Y., M. Deshmukh, A. D'Costa, J.A. Demaro, J.M. Gidday, A. Shah, Y. Sun, M.F. Jacquin, E.M. Johnson, and D.M. Holtzman. 1998. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J. Clin. Invest. 101:1992–1999. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous