Evidence that fibroblasts derive from epithelium during tissue fibrosis - PubMed (original) (raw)
Evidence that fibroblasts derive from epithelium during tissue fibrosis
Masayuki Iwano et al. J Clin Invest. 2002 Aug.
Abstract
Interstitial fibroblasts are principal effector cells of organ fibrosis in kidneys, lungs, and liver. While some view fibroblasts in adult tissues as nothing more than primitive mesenchymal cells surviving embryologic development, they differ from mesenchymal cells in their unique expression of fibroblast-specific protein-1 (FSP1). This difference raises questions about their origin. Using bone marrow chimeras and transgenic reporter mice, we show here that interstitial kidney fibroblasts derive from two sources. A small number of FSP1(+), CD34(-) fibroblasts migrate to normal interstitial spaces from bone marrow. More surprisingly, however, FSP1(+) fibroblasts also arise in large numbers by local epithelial-mesenchymal transition (EMT) during renal fibrogenesis. Both populations of fibroblasts express collagen type I and expand by cell division during tissue fibrosis. Our findings suggest that a substantial number of organ fibroblasts appear through a novel reversal in the direction of epithelial cell fate. As a general mechanism, this change in fate highlights the potential plasticity of differentiated cells in adult tissues under pathologic conditions.
Figures
Figure 1
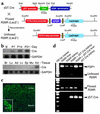
Characterization of γGT.Cre mice. (a) Plasmid map of the γGT.Cre transgene injected into B6 × SJL zygotes for the production of transgenic mice. (b) γGT.Cre transgenic mice express Cre transcripts only in the kidney and after postpartum day 7 (day P7), shown by Northern blot probed with cDNA encoding Cre and GAPDH. B, day of birth. (c) Immunofluorescent staining of a paraffin-embedded kidney section from γGT.Cre transgenic mice using antibody against Cre demonstrates staining in cortical proximal tubules but not in the medulla by confocal microscopy (×400); inset at higher power shows cortical tubular (CT) staining (×630). (d) R26R × γGT.Cre F1 hybrid mice express a recombination amplicon of approximately 575 bp’s (shown by PCR) (7, 8) in the kidney but not in the liver. bpA, poly A tail; Br, brain; Lu, lung; Ad, adrenal gland; Lv, liver; Sp, spleen; Mu, muscle; Kd, kidney.
Figure 2
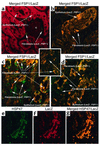
Confocal microscopy images of merged stainings from kidney tissue following UUO. (a) Glomeruli from the contralateral kidneys in R26R × γGT.Cre mice 10 days after unilateral UUO have no reaction product. Unfloxed LacZ+ tubular epithelium stained red with anti-LacZ antibodies as evidence of recombination, and floxed LacZ– interstitial fibroblasts formed before day P7 stained green (FSP1+) with anti-FSP1 antibodies. ×630. (b) A representative cortical tubule undergoing EMT in kidney harvested 10 days after UUO stained with anti-FSP1 (green) and anti-LacZ (red). The merged confocal image demonstrates unfloxed FSP1+, LacZ+ epithelial cells staining yellow as double-positive cells. The tubule is disaggregating, with cellular elements assuming the shape of new interstitial FSP1+, LacZ+ fibroblasts. ×630. (c) Renal cortical interstitium from kidney harvested 10 days after UUO demonstrated that floxed FSP1+, LacZ– fibroblasts formed before day P7 were stained green (FSP1+), and adjacent and newly formed unfloxed FSP1+, LacZ+ fibroblasts (those created after day P7) stained yellow (double positive for FSP1 and LacZ) after local EMT. ×400. (d) Tubular EMT in kidney cortical tissue harvested 10 days after UUO demonstrated that floxed FSP1+, LacZ– fibroblasts formed before day P7 were stained green (FSP1+), some cortical FSP1+, LacZ+ tubular epithelial cells undergoing EMT were yellow (double positive for FSP1 and LacZ), and adjacent and newly formed, unfloxed FSP1+, LacZ+ fibroblasts stained yellow after EMT. ×630. (e) Day 10 UUO kidney sections stained with anti-LacZ (green) and (f) anti-HSP47 (red). (g) Merged kidney section in yellow demonstrates colocalization of HSP47 (collagen type I production) in unfloxed tubular LacZ+, HSP47+ epithelium undergoing EMT and in new unfloxed LacZ+, HSP47+ fibroblasts. ×400.
Figure 3
Characterization of FSP1.GFP transgenic mice. (a) Plasmid map of the FSP1.GFP transgene injected into B6D2 zygotes for the production of transgenic mice. Ex 1, exon 1; In 1, intron 1. (b) Adult FSP1.GFP transgenic mice express GFP protein in all tissue fibroblasts. In adult FSP1.GFP mice, the eyes are demonstrably green under a Woods light compared with the wild-type because the GFP+ fibroblasts in the cornea cast a green tint. (c) RT-PCR screen of normal, whole-organ tissues for mRNA encoding GFP from GFP+ (+) or GFP– (–) littermates: skin, lungs, liver, kidney, heart/pericardium, thymus, and brain. (d) Green GFP+ fibroblasts from normal FSP1.GFP kidneys, liver, lung, and heart that costained with anti-FSP1 antibody (red) in their cytoplasm were occasionally observed in interstitial organ spaces; between tubules in kidney. ×630. (e) FACScan of FSP1.GFP bone marrow shows that 5–6% of FSP1+ marrow cells are GFP+ and that 95% of these FSP1+, GFP+ fibroblasts costain with anti-FSP1 antibody labeled with phycoerythrin (PE). Approximately 95% of GFP+ fibroblasts were also CD34–. (f) Decalcified bones from FSP1.GFP and GFP– wild-type mice were costained with propidium iodide, and GFP+ cells were identified in two locations by confocal microscopy, either scattered throughout the abluminal marrow as FSP1+, MSCs tethered to the sinusoidal network, or as FSP1+, GFP+ endosteal lining cells (BMLCs) adjacent to decalcified osteoid (B). ×630.
Figure 4
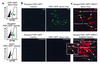
Bone marrow chimeras were produced by transfer of bone marrow from FSP1.GFP transgenic mice to Balb/c wild-type mice. After 30 days of recovery, they underwent surgical UUO; kidney and bone marrow were harvested 10 days later. (a) GFP+ donor marrow containing FSP1+ stromal fibroblasts (green) and representing 5–6% of the donor cell population on FACScan were found in chimeric recipients at harvest 10 days after UUO in approximately the same proportion as in wild-type donor marrow where there was no GFP expression. (b) Tissues harvested from chimeric mice following UUO of one kidney demonstrate a rare green FSP1+, GFP+ fibroblast in the interstitium of the normal, contralateral control kidney. More fibroblasts of this type appear in the kidney stressed by UUO and fibrogenesis ×400. FSP1.GFP transgenic mice were also subjected to UUO in parallel. These UUO kidneys also showed an increase in green FSP1+, GFP+ fibroblasts compared with the contralateral control (data not shown). (c) Merged confocal microscopy of kidney sections 10 days after UUO in donor or recipient mice demonstrates a mixture of GFP+, HSP47– fibroblasts (GFP shows as green), GFP–, HSP47+ fibroblasts (HSP47 shows as red), and double-labeled GFP+, HSP47+ fibroblasts (green/red or weak yellow). GFP–, HSP47+ tubular epithelial cells from UUO kidney tissue also stained red, reflecting their contribution to collagen type I production during EMT. ×630.
Figure 5
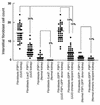
Fibroblast cell counts in renal tissues after UUO (8). Several different groups of kidneys were compared to determine the relative contributions of each to the source of fibroblasts. Forty to 80 random high-power fields in cortical kidneys were counted for FSP1+ fibroblasts, EMT-derived LacZ+ fibroblasts, or GFP+ fibroblasts, depending on the group. Mean fibroblast counts are represented by a horizontal line in each column: 12% of normal resident fibroblasts in the kidney come from bone marrow (number of FSP1+, GFP+ fibroblasts from normal, chimeric recipient kidneys divided by the number of FSP1+, GFP+ fibroblasts from FSP1.GFP+ donor kidneys); local EMT as a source is rare in the absence of fibrogenic stress (number of FSP1+, LacZ+ fibroblasts from normal kidneys divided by the number of FSP1+ fibroblasts from normal kidneys); and during experimental fibrosis, local EMT (number of FSP1+, LacZ+ fibroblasts from UUO kidneys divided by the number of FSP1+ fibroblasts from UUO kidneys) and bone marrow (number of FSP1+, GFP+ fibroblasts from UUO, chimeric recipient kidneys divided by the number of FSP1+ fibroblasts from UUO kidneys) contribute 36% and 15% of fibroblasts, respectively.
Comment in
- Renal interstitial fibrosis: remembrance of things past?
Herzlinger D. Herzlinger D. J Clin Invest. 2002 Aug;110(3):305-6. doi: 10.1172/JCI16377. J Clin Invest. 2002. PMID: 12163448 Free PMC article. Review. No abstract available.
Similar articles
- Renal interstitial fibrosis: remembrance of things past?
Herzlinger D. Herzlinger D. J Clin Invest. 2002 Aug;110(3):305-6. doi: 10.1172/JCI16377. J Clin Invest. 2002. PMID: 12163448 Free PMC article. Review. No abstract available. - Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation.
Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG. Okada H, et al. Kidney Int. 2000 Aug;58(2):587-97. doi: 10.1046/j.1523-1755.2000.00205.x. Kidney Int. 2000. PMID: 10916082 - Characterization of fibroblasts recruited from bone marrow-derived precursor in neonatal bronchopulmonary dysplasia mice.
Deng C, Wang J, Zou Y, Zhao Q, Feng J, Fu Z, Guo C. Deng C, et al. J Appl Physiol (1985). 2011 Jul;111(1):285-94. doi: 10.1152/japplphysiol.00201.2010. Epub 2011 Jan 13. J Appl Physiol (1985). 2011. PMID: 21233340 - Identification and characterization of a fibroblast marker: FSP1.
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Strutz F, et al. J Cell Biol. 1995 Jul;130(2):393-405. doi: 10.1083/jcb.130.2.393. J Cell Biol. 1995. PMID: 7615639 Free PMC article. - Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition.
Guarino M, Tosoni A, Nebuloni M. Guarino M, et al. Hum Pathol. 2009 Oct;40(10):1365-76. doi: 10.1016/j.humpath.2009.02.020. Epub 2009 Aug 19. Hum Pathol. 2009. PMID: 19695676 Review.
Cited by
- GLIPR-2 overexpression in HK-2 cells promotes cell EMT and migration through ERK1/2 activation.
Huang S, Liu F, Niu Q, Li Y, Liu C, Zhang L, Ni D, Pu X. Huang S, et al. PLoS One. 2013;8(3):e58574. doi: 10.1371/journal.pone.0058574. Epub 2013 Mar 13. PLoS One. 2013. PMID: 23516513 Free PMC article. - Phenolic secoiridoids in extra virgin olive oil impede fibrogenic and oncogenic epithelial-to-mesenchymal transition: extra virgin olive oil as a source of novel antiaging phytochemicals.
Vazquez-Martin A, Fernández-Arroyo S, Cufí S, Oliveras-Ferraros C, Lozano-Sánchez J, Vellón L, Micol V, Joven J, Segura-Carretero A, Menendez JA. Vazquez-Martin A, et al. Rejuvenation Res. 2012 Feb;15(1):3-21. doi: 10.1089/rej.2011.1203. Epub 2012 Jan 9. Rejuvenation Res. 2012. PMID: 22229524 Free PMC article. - Fsp1 cardiac embryonic expression delineates atrioventricular endocardial cushion, coronary venous and lymphatic valve development.
Cano-Ballesteros S, Palmquist-Gomes P, Marín-Sedeño E, Guadix JA, Pérez-Pomares JM. Cano-Ballesteros S, et al. J Anat. 2021 Feb;238(2):508-514. doi: 10.1111/joa.13306. Epub 2020 Sep 13. J Anat. 2021. PMID: 32920869 Free PMC article. - SMADS-Mediate Molecular Mechanisms in Sjögren's Syndrome.
Sisto M, Ribatti D, Lisi S. Sisto M, et al. Int J Mol Sci. 2021 Mar 21;22(6):3203. doi: 10.3390/ijms22063203. Int J Mol Sci. 2021. PMID: 33801157 Free PMC article. Review. - Immunohistochemical Localization of Epithelial Mesenchymal Transition Markers in Cyclosporine A Induced Gingival Overgrowth.
Arora H, Madapusi BT, Ramamurti A, Narasimhan M, Periasamy S, Rao SR. Arora H, et al. J Clin Diagn Res. 2016 Aug;10(8):ZC48-52. doi: 10.7860/JCDR/2016/20808.8271. Epub 2016 Aug 1. J Clin Diagn Res. 2016. PMID: 27656563 Free PMC article.
References
- Peifer M, McEwen DG. The ballet of morphogenesis: unveiling the hidden choreographers. Cell. 2002;109:271–274. - PubMed
- Watt FM, Hogan BLM. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. - PubMed
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. - PubMed
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1445. - PubMed
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials