Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin - PubMed (original) (raw)
Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin
Lisa L Gomez et al. J Neurosci. 2002.
Abstract
At the postsynaptic membrane of glutamatergic synapses, the cAMP-dependent protein kinase (PKA), protein kinase C (PKC), and calcineurin (CaN) anchoring protein AKAP79/150 is recruited to NMDA and AMPA glutamate receptors by postsynaptic density (PSD)-95 family membrane-associated guanylate kinase (MAGUK) scaffold proteins. These signaling scaffold complexes may function to regulate receptor phosphorylation in synaptic plasticity. Thus, it is important to understand regulation of AKAP79/150 targeting to synapses and recruitment to PSD-MAGUK complexes. AKAP79 is targeted to the plasma membrane by an N-terminal basic domain that binds phosphatidylinositol-4,5-bisphosphate (PI-4,5-P(2)) and is regulated by PKC phosphorylation and calmodulin binding. Here we demonstrate that this same domain also binds F-actin in a calmodulin- and PKC-regulated manner, targets to membrane ruffles enriched in F-actin and PI-4,5-P(2) in COS7 cells, and localizes to dendritic spines with F-actin and PSD-MAGUKs in hippocampal neurons. Inhibition of actin polymerization disrupted AKAP79 targeting of PKA and CaN to ruffles in COS7 cells and endogenous AKAP79/150 dendritic spine localization with PKA, CaN, and PSD-MAGUKs in neurons. AKAP79/150 postsynaptic localization was rapidly regulated by NMDA receptors through CaN activation and F-actin remodeling, further suggesting that AKAP79/150 signaling scaffold targeting depends on actin dynamics. NMDA receptor activation also regulated dendritic spine localization of PKA and CaN and association of the AKAP79/150-PKA complex with PSD-MAGUKs. Because AMPA receptor PKA phosphorylation and synaptic localization are regulated by similar NMDA receptor-CaN signaling pathways linked to hippocampal long-term depression, this regulation of AKAP79/150 postsynaptic targeting might be important for synaptic plasticity.
Figures
Fig. 1.
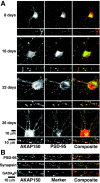
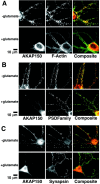
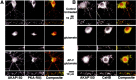
Postsynaptic localization of AKAP79/150 at excitatory synapses in hippocampal neurons.A, Developmental regulation of AKAP79/150 colocalization with the MAGUK PSD-95. Rat neonatal hippocampal neurons cultured at low density for the indicated number of days were stained for AKAP150 (red) and the postsynaptic density MAGUK PSD-95 (green). The small panels are magnifications of dendrites. B, Punctate colocalization of AKAP79/150 with PSD-95 on dendritic spines: proximity of AKAP79/150 puncta to presynaptic terminals stained for synapsin but not inhibitory postsynaptic elements stained for GABAA receptors. Neurons (2–3 weeks old) cultured at medium-high density were stained with anti-rat AKAP150 (red) and anti-PSD-95, anti-synapsin, or anti-GABAA receptor (all in green). Codistribution is seen as yellow-orange in composite images. The red and green arrowheads point to puncta for AKAP150 and PSD-95, respectively, that are not colocalized even in “mature” dendrites. The images shown are representative of multiple neurons imaged in more than three experiments for each condition.
Fig. 2.
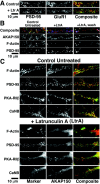
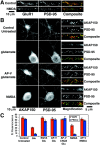
AKAP79/150 colocalization with F-actin, PSD-95, PKA, and calcineurin on dendritic spines depends on the actin cytoskeleton: disruption with latrunculin A. A, AMPA receptor GluR1 (red) dendritic colocalization with PSD-95 (green) is disrupted by pretreatment with latrunculin A (+LtrA; 5 μ
m
, 2 hr).B, Dendritic spine colocalization in hippocampal neurons (seen as white in Composite panels) of AKAP79/150 (red) with F-actin (green) and PSD-95 (blue) is disrupted by latrunculin A treatment (2 μ
m
; 2 hr) and is reversible after drug washout and recovery (16 hr). C, Dendritic spine colocalization of AKAP79/150 (red) with CaN and PKA as well as PSD-95 and F-actin (markers; all in_green_) is disrupted by latrunculin A (5 μ
m
, 2 hr). Magnifications of dendrites are shown. See Results for quantitation.
Fig. 3.
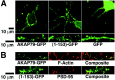
The AKAP79 N-terminal basic domain binds F-actin_in vitro_ and localizes with PI-4,5-P2 and cortical F-Actin in COS7 cells: targeting of PKA and CaN to membrane ruffles. A, Diagram showing the structures of AKAP79WT(1–427), (1–153), and (154–427) proteins used for actin-binding studies. Locations of the three (A,B, C) basic membrane targeting/phospholipid binding regions and the CaNA and PKA-RII anchoring domains are indicated. B, AKAP79WT and an N-terminal (1–153) fragment but not a C-terminal (154–427) fragment bind to F-actin in vitro. Purified full-length AKAP79WT, (1–153), or (154–427) fragments were incubated with (+) or without (−) purified actin in buffers favoring monomeric G-actin (G) or F-actin polymerization (F). Binding to polymerized F-actin was detected as sedimentation of AKAP79 immunoreactivity in pellet fractions (P) after centrifugation. The data shown are representative of results obtained in multiple (more than three) independent experiments. C, AKAP79–GFP colocalizes with cortical/membrane F-actin in COS7 cells. In control untreated COS7 cells, overlap of AKAP79–GFP (green) and F-actin (TxRd–Phalloidin; red) in plasma membrane ruffles is seen as yellow-orange in the composite image. Treatment of AKAP79–GFP-transfected COS7 cells with the actin polymerization inhibitor cytochalasin D (5 μ
m
, 4–5 hr) (+CHD) disrupts both the actin cytoskeleton and AKAP79–GFP/F-actin colocalization in membrane ruffles. D, The AKAP79(1–153)–GFP (green) N-terminal targeting domain also colocalizes with cortical F-actin (red) ruffles in a cytochalasin D (+CHD)-sensitive manner. E, The GFP–AKAP79(150–427) (green) C-terminal fragment containing CaNA and PKA anchoring domains but lacking the basic targeting domains is localized to the cytoplasm and not colocalized with cortical F-actin (red) in COS7 cells. PKA-RII–GFP (F, green) and mycCaNA (anti-myc; G,red) are found in the cytoplasm when expressed alone in COS7 cells. H, Coexpression of AKAP79 (anti-79, red) targets PKA-RII–GFP (green) to plasma membrane ruffles in a cytochalasin D (+CHD)-sensitive manner.I, Coexpression of AKAP79–GFP (green) targets mycCaNA (anti-myc; red) to plasma membrane ruffles in a cytochalasin D (+CHD)-sensitive manner. J, Colocalization (blue-green) of AKAP79–CFP (blue) with PI-4,5-P2 detected by PLCδ-PH–YFP (green) in membrane ruffles of living COS7 cells.K, Colocalization (blue-green) of the AKAP79(1–153)-CFP N-terminal targeting domain (blue) with PI-4,5-P2 detected by PLCδ-PH–YFP (green) in membrane ruffles of living COS7 cells. The images shown in C_–_K are representative of multiple cells imaged in three independent experiments for each condition. TxRd, Texas Red.
Fig. 4.
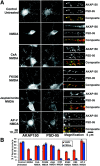
The AKAP79 N-terminal basic domain targets to dendritic spines in hippocampal neurons. A, Targeting of AKAP79–GFP and the N-terminal basic domain (1–153)–GFP but not GFP alone (all in green) to dendritic spines in transfected neurons. B, Colocalization of AKAP79–GFP and (1–153)–GFP (both green) with F-actin and PSD-95 (both red) on dendritic spines. See Results and Materials and Methods for quantitation and details.
Fig. 5.
Regulation of AKAP79 targeting domain F-actin binding by phosphorylation and Ca2+-calmodulin binding. A, Regulation of AKAP79(1–153) F-actin binding by Ca2+–CaM. AKAP79(1–153) was incubated for 10 min as indicated with (+) or without (−) CaM (1 μ
m
), Ca2+ (100 μ
m
), or EGTA (5 m
m
) before assaying for F-actin binding by cosedimentation. B, Regulation of AKAP79(1–153) F-actin binding by PKC phosphorylation. AKAP79(1–153) was PKC phosphorylated before assaying for F-actin binding by cosedimentation. Control incubations were incubated in the absence of kinase. C, Competition between Ca2+–CaM binding and PKC phosphorylation of the AKAP79(1–153) targeting domain. AKAP79(1–153) was phosphorylated with [γ−32P]ATP by PKC (Control), with Ca2+–CaM or CaM and EGTA present.D, Previous incubation with F-actin inhibits regulation of AKAP79(1–153) F-actin binding by PKC phosphorylation and Ca2+–CaM binding. In the top panel, AKAP79(1–153) was either phosphorylated by PKC before incubation with F-actin (Actin 2nd; as in B) or incubated first with F-actin and then with PKC (Actin 1st) followed by assaying for F-actin cosedimentation. In the bottom panel, the AKAP79(1–153) targeting domain fragment either was incubated with Ca2+–CaM before incubation with F-actin (Actin 2nd; as in A) or was incubated first with F-actin and then with Ca2+–CaM (Actin 1st) followed by assaying for F-actin cosedimentation. The data shown are representative of results obtained in at least three independent experiments. A, B, and D are immunoblots (IB:); C is an autoradiogram (P-32).
Fig. 6.
Glutamate regulation of AKAP79/150 postsynaptic localization and dendritic spine F-actin in hippocampal neurons. A, Redistribution of AKAP79/150 and reorganization of dendritic F-actin in response to glutamate in hippocampal neurons. B, Loss of AKAP79/150 colocalization with postsynaptic PSD-95 family MAGUK scaffolds after glutamate treatment. C, Glutamate regulation of AKAP79/150 localization near presynaptic terminals marked by synapsin. Neurons were treated for 10 min with (+) or without (−) glutamate (50 μ
m
) before staining for AKAP150 (red), F-actin–phalloidin, PSD-95 family members (including PSD-95 and SAP97), or synapsin (all in green). The images shown are representative of neurons imaged in multiple (>3) experiments for each condition.
Fig. 7.
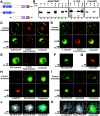
Regulation of AKAP79/150 postsynaptic localization by NMDA receptor activation. A, Loss of GluR1 AMPA-receptor (red) dendritic colocalization with PSD-95 (green) in NMDA-treated neurons.B, Loss of AKAP79/150 (red) colocalization with PSD-95 (green) in glutamate or NMDA-treated neurons. Neurons were treated for 10 min with glutamate (50 μ
m
) or NMDA (50 μ
m
). Glutamate regulation of AKAP79/150 targeting was blocked by the NMDAR antagonist AP-V (100 μ
m
, 15 min pretreatment). Large panels show AKAP150 and PSD-95 localization separately in both dendrites and soma. The Magnification panels show AKAP150, PSD-95, and Composite_images for localization in single dendrites to show details better.C, Quantitation of AKAP79/150 mean fluorescence intensity distributions between dendrites and somata (%D/S_, red) and integrated colocalization with PSD-95 (PSD co,blue) from multiple experiments for the treatment conditions in B as well additional conditions described below. See Results and Materials and Methods for additional details. Glutamate regulation of AKAP79/150 targeting was also blocked by chelation of extracellular Ca2+ with EGTA (5 m
m
). In the presence of glutamate and AP-V, no additional effect of the AMPAR antagonist CNQX (50 μ
m
, 15 min pretreatment) was seen. Selective activation of AMPA receptors with 50 μ
m
AMPA (in the presence of 100 μ
m
AP-V), although having some effect on AKAP79/150 postsynaptic localization, did not result in a dramatic redistribution as seen with NMDA or glutamate (data not shown). NMDA regulation of AKAP79/150 targeting was also blocked by the NMDAR antagonist AP-V (100 μ
m
, 15 min pretreatment).
Fig. 8.
Activation of the protein phosphatase-2B calcineurin and reorganization of F-actin are necessary for NMDA receptor regulation of AKAP79/150 postsynaptic localization.A, Activation of CaN and reorganization of F-actin are necessary for NMDA regulation of AKAP79/150 localization. Neurons were left untreated or pretreated with the indicated CaN–PP2B phosphatase inhibitors CsA (1 μ
m
, 30 min) or FK506 (1 μ
m
, 30 min), the actin-stabilizing drug jasplakinolide (2 μ
m
, 2 hr), or the NMDA receptor antagonist AP-V (100 μ
m
, 30 min) before NMDA treatment (50 μ
m
, 10 min). The neurons were then fixed and stained for AKAP79/150 (red) and PSD-95 (green).Large panels show AKAP150 and PSD-95 localization separately in both dendrites and soma. The Magnification panels show AKAP150, PSD-95, and Composite_images for localization in single dendrites to show details better.B, Calcineurin protein phosphatase but not protein kinase activity is necessary for NMDA regulation of AKAP79/150 localization. Quantitation of AKAP79/150 mean fluorescence intensity distributions between dendrites and somata (%D/S_,red) and integrated colocalization with PSD-95 (PSDco, blue) from multiple experiments for the treatment conditions in _A_as well additional conditions described below are shown. See Results and Materials and Methods for additional details. Neurons were also pretreated for 30 min with the indicated kinase inhibitors for PKA, H-89 (500 n
m
); PKC, CHE (10 μ
m
); or CaMKII, KN-62 (5 μ
m
) before NMDA treatment (50 μ
m
, 10 min). Pretreaments with casein kinase II inhibitor (DRB, 50–100 μ
m
) or multiple kinase inhibitors (H-89, CHE, KN-62, DRB) had no effects on AKAP150 redistribution in NMDA-treated cells (data not shown).
Fig. 9.
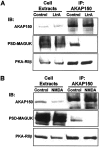
Regulation of AKAP79/150-PKA association with PSD–MAGUKs by inhibition of actin polymerization and NMDA receptor activation. A, Inhibiting F-actin polymerization with latrunculin A disrupts association of AKAP79/150 with PSD–MAGUKs but not PKA in complexes isolated from hippocampal neuron cell extracts.B, NMDA receptor activation disrupts association of AKAP79/150 with PSD–MAGUKs but not PKA in complexes isolated from hippocampal neuron cell extracts. Neurons grown at high density on Petri plates were either untreated for control conditions or treated with latrunculin A (+LtrA; 5 μ
m
, 4 hr) (A) or NMDA (50 μ
m
, 10 min) (B) before harvesting and lysis to prepare whole-cell extracts. AKAP79/150 was then immunoprecipitated (IP: AKAP150) from Triton X-100-soluble fractions prepared from these extracts. The immunoprecipitates and whole-cell extracts were then analyzed by immunoblotting (IB:) for AKAP150, PSD-MAGUK family members, and the PKA-RIIβ regulatory subunit as indicated. The data shown are representative of three experiments done on two to four samples for each condition.
Fig. 10.
NMDA receptor activation regulates dendritic spine localization of PKA and CaN with AKAP79/150.A, NMDA receptor regulation of PKA-RIIβ and AKAP79/150 localization. B, Disruption of dendritic spine colocalization of CaNB and AKAP79/150 in response to NMDA receptor activation. Neurons (2–3 weeks old) were untreated, pretreated with antagonist AP-V (100 μ
m
, 30 min), or treated with glutamate (50 μ
m
, 10 min) as indicated. Control untreated and treated neurons were stained for AKAP79/150 (red) and either PKA-RIIβ regulatory subunits (green) in A or CaNB (green) in_B_. The smaller panels are magnifications of dendrites. See Results for quantitation.
Fig. 11.
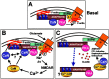
Possible relationships in regulation of the AKAP79/150 scaffold and AMPA receptors by NMDA receptor signaling pathways. A_–_C, Implications for LTD: a model of possible roles for NMDA receptors and CaN-mediated F-actin reorganization in regulation of AKAP79/150 localization and AMPA receptor phosphorylation and localization. For the sake of simplicity, only AMPA receptor–SAP97–AKAP79/150 complexes are shown. Nearby NMDA receptor–PSD-95–AKAP79/150 complexes are also likely to participate in these signaling events. See Discussion for details.
Similar articles
- Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review. - cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein.
Smith KE, Gibson ES, Dell'Acqua ML. Smith KE, et al. J Neurosci. 2006 Mar 1;26(9):2391-402. doi: 10.1523/JNEUROSCI.3092-05.2006. J Neurosci. 2006. PMID: 16510716 Free PMC article. - Regulation of postsynaptic structure and function by an A-kinase anchoring protein-membrane-associated guanylate kinase scaffolding complex.
Robertson HR, Gibson ES, Benke TA, Dell'Acqua ML. Robertson HR, et al. J Neurosci. 2009 Jun 17;29(24):7929-43. doi: 10.1523/JNEUROSCI.6093-08.2009. J Neurosci. 2009. PMID: 19535604 Free PMC article. - Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression.
Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Tavalin SJ, et al. J Neurosci. 2002 Apr 15;22(8):3044-51. doi: 10.1523/JNEUROSCI.22-08-03044.2002. J Neurosci. 2002. PMID: 11943807 Free PMC article. - CaMKII regulates the depalmitoylation and synaptic removal of the scaffold protein AKAP79/150 to mediate structural long-term depression.
Woolfrey KM, O'Leary H, Goodell DJ, Robertson HR, Horne EA, Coultrap SJ, Dell'Acqua ML, Bayer KU. Woolfrey KM, et al. J Biol Chem. 2018 Feb 2;293(5):1551-1567. doi: 10.1074/jbc.M117.813808. Epub 2017 Dec 1. J Biol Chem. 2018. PMID: 29196604 Free PMC article. Review.
Cited by
- Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels.
Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA. Oliveria SF, et al. J Neurosci. 2012 Oct 31;32(44):15328-37. doi: 10.1523/JNEUROSCI.2302-12.2012. J Neurosci. 2012. PMID: 23115171 Free PMC article. - SPIN90 dephosphorylation is required for cofilin-mediated actin depolymerization in NMDA-stimulated hippocampal neurons.
Cho IH, Lee MJ, Kim DH, Kim B, Bae J, Choi KY, Kim SM, Huh YH, Lee KH, Kim CH, Song WK. Cho IH, et al. Cell Mol Life Sci. 2013 Nov;70(22):4369-83. doi: 10.1007/s00018-013-1391-4. Epub 2013 Jun 14. Cell Mol Life Sci. 2013. PMID: 23765104 Free PMC article. - Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace.
Mao SC, Hsiao YH, Gean PW. Mao SC, et al. J Neurosci. 2006 Aug 30;26(35):8892-9. doi: 10.1523/JNEUROSCI.0365-06.2006. J Neurosci. 2006. PMID: 16943544 Free PMC article. - A dynamic interface between ubiquitylation and cAMP signaling.
Rinaldi L, Sepe M, Donne RD, Feliciello A. Rinaldi L, et al. Front Pharmacol. 2015 Sep 4;6:177. doi: 10.3389/fphar.2015.00177. eCollection 2015. Front Pharmacol. 2015. PMID: 26388770 Free PMC article. Review. - Spine remodeling and synaptic modification.
Wang XB, Zhou Q. Wang XB, et al. Mol Neurobiol. 2010 Feb;41(1):29-41. doi: 10.1007/s12035-009-8093-9. Epub 2010 Jan 6. Mol Neurobiol. 2010. PMID: 20049655 Review.
References
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. - PubMed
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous