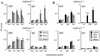Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barré syndrome - PubMed (original) (raw)
Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barré syndrome
Tyrone Bowes et al. Infect Immun. 2002 Sep.
Abstract
Guillain-Barré syndrome following Campylobacter jejuni infection is frequently associated with anti-ganglioside autoantibodies mediated by molecular mimicry with ganglioside-like oligosaccharides on bacterial lipopolysaccharide (LPS). The regulation of antibody responses to these T-cell-independent antigens is poorly understood, and only a minority of Campylobacter-infected individuals develop anti-ganglioside antibodies. This study investigates the response to gangliosides and LPS in strains of mice by using a range of immunization strategies. In normal mice following intraperitoneal immunization, antibody responses to gangliosides and LPS are low level but can be enhanced by the antigen format or coadministration of protein to recruit T-cell help. Class switching from the predominant immunoglobulin M (IgM) response to IgG3 occurs at low levels, suggesting B1-cell involvement. Systemic immunization results in poor responses. In GalNAc transferase knockout mice that lack all complex gangliosides and instead express high levels of GM3 and GD3, generation of anti-ganglioside antibodies upon immunization with either complex gangliosides or ganglioside-mimicking LPS is greatly enhanced and exhibits class switching to T-cell-dependent IgG isotypes and immunological memory, indicating that tolerance to self gangliosides is a major regulatory factor. Responses to GD3 are suppressed in knockout mice compared with wild-type mice, in which responses to GD3 are induced specifically by GD3 and as a result of polyclonal B-cell activation by LPS. The anti-ganglioside response generated in response to LPS is also dependent on the epitope density of the ganglioside mimicked and can be further manipulated by providing secondary signals via lipid A and CD40 ligation.
Figures
FIG. 1.
Oligosaccharide structures. The whole structure of GQ1b is shown, but this has not been found in C. jejuni. NeuAc, _N_-acetyl neuraminic acid; X, Glc (1→1) ceramide (gangliosides) or the remaining core OS-lipid A (LPSs).
FIG. 2.
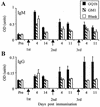
Anti-ganglioside IgM (A) and IgG (B) responses in BALB/c mice (n = 3) following i.p. immunization with GQ1b-ova liposomes. The error bars indicate SD. The mice were primed with ova-alum and then immunized on day 7 with GQ1b-ova liposomes and bled 4 and 14 days later. The mice were reimmunized 2 and 4 weeks following the initial immunization and rebled 4 and 11 days later. A diminishing IgM response and rising IgG response to GQ1b can be clearly seen upon repeated immunization. The IgG subclass of the anti-GQ1b response was exclusively IgG3 (mean OD at 1:200 serum dilution, 0.61 ± 0.16 SD).
FIG. 3.
Anti-ganglioside antibody responses in GalNAcT−/− and GalNAcT+/+ mice following i.p. immunization with GD1a-ova liposomes (A) and GQ1b-ova liposomes (B). The error bars indicate SD. Group sizes were as follows: GD1a-ova liposomes, n = 8 (GalNAcT−/−) and n = 5 (GalNAcT+/+); GQ1b-ova liposomes, n = 5 (GalNAcT−/−) and n = 3 (GalNAcT+/+). The mice were primed with ova-alum and immunized on day 7 with GQ1b-ova or GD1a-ova liposomes. The mice were further immunized at 2-week intervals and bled 4 days after each immunization. Elevated IgG anti-GD1a antibody titers are evident in GalNAcT−/− mice compared with GalNAcT+/+ mice, and they increase upon subsequent immunization (*, P < 0.005 after the second immunization). Elevated IgG anti-GQ1b antibody titers are evident in GalNAcT−/− mice compared with GalNAcT+/+ mice (**, P < 0.001 after the second immunization).
FIG. 4.
Anti-ganglioside antibody responses in GalNAcT+/+ (A) and GalNAcT−/− (B) mice (two groups; n = 5) following repeated i.p. immunizations with GD3-ova liposomes. The error bars indicate SD. The mice were primed with ova-alum, immunized on day 7 with GD3-ova liposomes, and bled 4 days later. The mice were reimmunized at 2-week intervals and bled 4 days after each immunization. Anti-GD3 IgM and IgG antibody responses are depressed in the GD3-rich GalNAcT−/− mice compared with GalNAcT+/+ mice, but this did not reach statistical significance (P = 0.08 post-third immunization for IgM, and P = 0.3 for IgG).
FIG. 5.
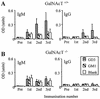
Anti-ganglioside antibody responses in GalNAcT−/− and GalNAcT+/+ mice after i.p. immunization with HS:4 LPS (A) or HS:19(GM1+ GT1a+) LPS (B) in CFA and subsequently at 2-week intervals with LPS in IFA. The error bars indicate SD. The group sizes were as follows: HS:4 group, n = 9 (GalNAcT−/− mice) and n = 7 (GalNAcT+/+ mice); HS:19(GM1+ GT1a+) group, n = 5 (GalNAcT−/− mice) and n = 3 (GalNAcT+/+ mice). All sera were screened 4 days after each immunization. IgG anti-ganglioside antibodies, corresponding to the core OS structure on the immunizing LPS [GD1a for HS:4 LPS; GT1a and GM1 for HS:19(GM1+ GT1a+) LPS], appear after the second and third immunizations in significantly greater amounts in GalNAcT−/− than in GalNAcT+/+ mice (*, P < 0.05). In the ganglioside ELISA, GQ1b is substituted for GT1a (see Materials and Methods for details).
FIG. 6.
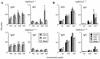
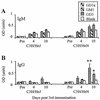
Anti-ganglioside IgM (A) and IgG (B) antibody responses following HS:4 LPS immunization of C3H/HeN and C3H/HeJ (LPS low responder) mice. The error bars indicate SD. HS:4 LPS bears a GD1a-like epitope. Groups of mice (n = 5) received serial immunizations with HS:4 LPS in CFA-IFA at 2-week intervals, and sera were assayed on days 4 and 10 following each immunization. C3H/HeN mice showed significantly greater IgG responses than C3H/HeJ mice to GD3 (*, P = 0.002), and elevated IgG responses to GD1a, compared with C3H/HeJ mice (**, P = 0.066).
FIG. 7.
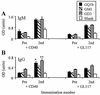
. Anti-ganglioside IgM (A) and IgG (B) antibody responses in BALB/c mice immunized with HS:19(GM1+ GT1a+) LPS coadministered with anti-CD40 antibody or control antibody, GL117. Groups of BALB/c mice (n = 4) were immunized i.p. with HS:19(GM1+ GT1a+) LPS and anti-CD40 antibody or GL117 and then reimmunized on day 21 and bled on day 28 (7 days post-second immunization). Mice treated with anti-CD40 antibody showed slightly greater anti-GQ1b and anti-GD3 responses than control antibody-treated mice (*, P < 0.005; **, P < 0.05), but there was no significant difference in anti-GM1 antibody responses.
Similar articles
- Ganglioside mimicry of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity in rabbits.
Ang CW, Noordzij PG, de Klerk MA, Endtz HP, van Doorn PA, Laman JD. Ang CW, et al. Infect Immun. 2002 Sep;70(9):5081-5. doi: 10.1128/IAI.70.9.5081-5085.2002. Infect Immun. 2002. PMID: 12183556 Free PMC article. - [Campylobacter jejuni enteritis and Guillain-Barré syndrome].
Yuki N. Yuki N. Rinsho Byori. 1999 Aug;47(8):713-8. Rinsho Byori. 1999. PMID: 10511801 Review. Japanese. - Guillain-Barré syndrome-related Campylobacter jejuni in Bangladesh: ganglioside mimicry and cross-reactive antibodies.
Islam Z, Gilbert M, Mohammad QD, Klaij K, Li J, van Rijs W, Tio-Gillen AP, Talukder KA, Willison HJ, van Belkum A, Endtz HP, Jacobs BC. Islam Z, et al. PLoS One. 2012;7(8):e43976. doi: 10.1371/journal.pone.0043976. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952833 Free PMC article. - Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations.
Goodyear CS, O'Hanlon GM, Plomp JJ, Wagner ER, Morrison I, Veitch J, Cochrane L, Bullens RW, Molenaar PC, Conner J, Willison HJ. Goodyear CS, et al. J Clin Invest. 1999 Sep;104(6):697-708. doi: 10.1172/JCI6837. J Clin Invest. 1999. PMID: 10491405 Free PMC article.
Cited by
- Axolemmal nanoruptures arising from paranodal membrane injury induce secondary axon degeneration in murine Guillain-Barré syndrome.
Cunningham ME, McGonigal R, Barrie JA, Campbell CI, Yao D, Willison HJ. Cunningham ME, et al. J Peripher Nerv Syst. 2023 Mar;28(1):17-31. doi: 10.1111/jns.12532. Epub 2023 Feb 12. J Peripher Nerv Syst. 2023. PMID: 36710500 Free PMC article. - Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies.
Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, Kepler TB, Alam SM, Liao HX, Cain DW, Spicer L, VandeBerg JL, Haynes BF, Kelsoe G. Yang G, et al. J Exp Med. 2013 Feb 11;210(2):241-56. doi: 10.1084/jem.20121977. Epub 2013 Jan 28. J Exp Med. 2013. PMID: 23359068 Free PMC article. - HIV-1 Envelope Mimicry of Host Enzyme Kynureninase Does Not Disrupt Tryptophan Metabolism.
Bradley T, Yang G, Ilkayeva O, Holl TM, Zhang R, Zhang J, Santra S, Fox CB, Reed SG, Parks R, Bowman CM, Bouton-Verville H, Sutherland LL, Scearce RM, Vandergrift N, Kepler TB, Moody MA, Liao HX, Alam SM, McLendon R, Everitt JI, Newgard CB, Verkoczy L, Kelsoe G, Haynes BF. Bradley T, et al. J Immunol. 2016 Dec 15;197(12):4663-4673. doi: 10.4049/jimmunol.1601484. Epub 2016 Nov 14. J Immunol. 2016. PMID: 27849170 Free PMC article. - Redemption of autoreactive B cells.
Haynes BF, Verkoczy L, Kelsoe G. Haynes BF, et al. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9022-3. doi: 10.1073/pnas.1407877111. Epub 2014 Jun 11. Proc Natl Acad Sci U S A. 2014. PMID: 24920593 Free PMC article. No abstract available. - Anti-ganglioside antibodies are removed from circulation in mice by neuronal endocytosis.
Cunningham ME, McGonigal R, Meehan GR, Barrie JA, Yao D, Halstead SK, Willison HJ. Cunningham ME, et al. Brain. 2016 Jun;139(Pt 6):1657-65. doi: 10.1093/brain/aww056. Epub 2016 Mar 26. Brain. 2016. PMID: 27017187 Free PMC article.
References
- Alfonso, M., and J. Zeuthen. 1996. Generation of human monoclonal antibodies against ganglioside antigens and their applications in the diagnosis and therapy of cancer. Acta Oncol. 35:287-295. - PubMed
- Allos, B. M. 1997. Association between Campylobacter infection and Guillain-Barre syndrome. J. Infect. Dis. 176(Suppl. 2):S125-S128. - PubMed
- Anderson, K. V. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12:13-19. - PubMed
- Ang, C. W., M. A. De Klerk, H. P. Endtz, B. C. Jacobs, J. D. Laman, F. G. van der Meche, and P. A. van Doorn. 2001. Guillain-Barre syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b antibodies in rabbits. Infect. Immun. 69:2462-2469. - PMC - PubMed
- Ang, C. W., M. Koga, B. C. Jacobs, N. Yuki, F. G. van der Meche, and P. A. van Doorn. 2001. Differential immune response to gangliosides in Guillain-Barre syndrome patients from Japan and The Netherlands. J. Neuroimmunol. 121:83-87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials