Basolateral targeting of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal but independent of the C-terminal ERBIN-binding domain - PubMed (original) (raw)
Basolateral targeting of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal but independent of the C-terminal ERBIN-binding domain
Christian Dillon et al. Mol Cell Biol. 2002 Sep.
Abstract
ERBB2 is a receptor tyrosine kinase present on the basolateral membrane of polarized epithelia and has important functions in organ development and tumorigenesis. Using mutagenic analyses and Madin-Darby canine kidney (MDCK) cells, we have investigated the signals that regulate basolateral targeting of ERBB2. We show that basolateral delivery of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal residing between Gln-692 and Thr-701. The signal shows only limited sequence homology to known basolateral targeting signals and is both necessary and sufficient for correct sorting of ERBB2. In addition we demonstrate that this motif can function as a dominant basolateral targeting signal by its ability to redirect the apically localized P75 neurotrophin receptor to the basolateral membrane domain of polarized epithelial cells. Interestingly, LLC-PK1 cells, which are deficient for the micro 1B subunit of the AP1B adaptor complex, missort a large proportion of ERBB2 to the apical membrane domain. This missorting can be partially corrected by the introduction of micro 1B, suggesting a possible role for AP1B in ERBB2 endosomal trafficking. Furthermore, we find that the C-terminal ERBIN binding domain of ERBB2 is not necessary for its basolateral targeting in MDCK cells.
Figures
FIG. 1.
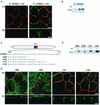
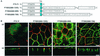
ERBB2 contains a juxtamembrane basolateral sorting signal. (A) MDCK cells containing an inducible ERBB2 expression vector were grown on transwell filters to confluence before overnight induction with tetracycline (1 μg/ml). After induction, cells were fixed in methanol, incubated with a rat antibody to ZO-1 and a mouse monoclonal antibody against ERBB2, and subsequently stained with FITC-conjugated goat anti-mouse IgG and TRITC-conjugated goat anti-rat IgG. Panels show confocal images of the XY sections through the tight junctions, and XZ sections with the apical membrane uppermost. (B) Immunoblot of an MDCK cell clone showing induced and uninduced ERBB2 expression. tet, tetracycline. (C) Schematic depiction of ERBB2 vectors showing the transmembrane domain (TM) and the extent of the C-terminal truncations in the juxtamembrane domain (amino acids 680 to 710). The asterisk shows the position of the introduced termination codon. (D) Immunoblot analysis of truncation mutants analyzed by confocal microscopy in panel E. (E) Confocal microscopy images of XY sections and XZ sections prepared and stained as for panel A. Bars, 5 μm.
FIG. 2.
Quantification of steady-state cell surface ERBB2. Stable clones of MDCK cells expressing full-length ERBB2 and truncation mutants Δ690, Δ695, Δ701, and Δ706 were grown as described in the legend to Fig. 1. To quantitate the surface expression of ERBB2, apical (Ap) or basolateral (BL) membrane proteins were biotinylated, captured on immobilized streptavidin beads, and then prepared for immunoblotting (see Materials and Methods). Error bars, standard errors.
FIG. 3.
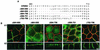
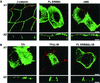
The basolateral and apical localization of ERBB2 proteins containing juxtamembrane internal deletions. (A) Schematic depiction of ERBB2 juxtamembrane domain showing the extents of internal cytoplasmic deletions for mutations Δ684-690, Δ684-695, Δ684-706, Δ696-701, and Δ702-706. (B) Clones of MDCK cells expressing the mutations shown in panel A. Cells were processed and stained as described in the legend to Fig. 1. Bars, 5 μm.
FIG. 4.
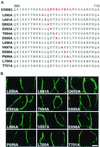
Membrane distribution of ERBB2 proteins containing single amino acids replaced with alanine in polarized MDCK cells. (A) Sequence of the ERBB2 juxtamembrane region showing the positions of the alanine substitutions within the described basolateral sorting motif (red). (B) MDCK cells were transiently transfected with expression vectors containing the mutations shown in panel A, and then the cells were processed and stained as described in the legend to Fig. 1. Panels show vertical XZ sections with FITC staining of ERBB2 and TRITC staining of ZO-1. Bar, 5 μm.
FIG. 5.
Apical and basolateral distribution of ERBB2 proteins containing multiple amino acid substitutions in polarized MDCK cells. (A) Sequence of the ERBB2 juxtamembrane targeting signal (red) showing positions of multiple alanine substitutions. (B) MDCK cells were transiently transfected with expression vectors containing the mutations shown in panel A, and then the cells were processed and stained as described in the legend to Fig. 1. Panels show confocal images of XY sections through the tight junctions and XZ sections with the apical membrane uppermost. Bars, 5 μm.
FIG. 6.
Apical and basolateral location of P75NTR/ERBB2 chimeras in MDCK cells. (A) Depiction of the P75NTR/ERBB2 chimeras tested in polarized MDCK cells. The extracellular and transmembrane domains of P75NTR were fused in frame either to the full-length cytoplasmic domain of ERBB2 or to truncations similar to those shown in Fig. 1. (B) MDCK cell clones containing inducible P75NTR/ERBB2 chimeras were grown on transwell filters to confluence before induction with tetracycline (1 μg/ml). Induced cells were processed and stained as described in the legend to Fig. 1, except that the ERBB2 antibody was replaced by an antibody against P75NTR. Panels show confocal images of XY sections through the tight junctions and XZ sections with the apical membrane uppermost. Bars, 5 μm.
FIG. 7.
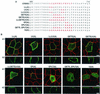
Apical localization of ERBB2 in LLC-PK1 cells can be partially rescued with μ1B. (A) LLC-PK1 cells were transiently transfected with a full-length ERBB2 expression vector or a similar vector containing the C-terminal truncation Δ690 (Fig. 1C). After 2 days, cells were fixed in paraformaldehyde and were incubated with a rat monoclonal antibody to ZO-1 and mouse monoclonal antibodies to either β-catenin or ERBB2 as indicated. The markers for tight junctions and lateral staining, ZO1 and β-catenin, respectively, confirm that the LLC-PK1 cells were polarized. The Δ690 mutant shows an exclusively apical localization similar to that in MDCK cells (Fig. 1E). (B) LLC-PK1 cells were transiently transfected with a transferrin receptor (TFr) or a full-length ERBB2 expression vector with or without a μ1B expression vector. Panels show confocal images of basolaterally localized transferrin receptor or ERBB2 in the presence of μ1B, although some cells still show a mixed localization. Bars, 5 μm.
FIG. 8.
Mutation analysis of the C-terminal ERBIN binding domain of ERBB2. (A) Schematic depiction of the ERBB2 protein and the C-terminal mutations within the ERBIN binding domain. (B) MDCK cell clones containing inducible mutated ERBB2 expression vectors as shown in panel A were grown on transwell filters to confluence before induction with tetracycline (1 μg/ml). Induced cells were processed and stained as described in the legend to Fig. 1. (C) Schematic depiction of a chimera composed of the extracellular and transmembrane domains of P75NTR fused in frame to the ERBIN binding domain of ERBB2 with or without alanine substitution mutations as shown. (D) Localization of the chimeras diagrammed in panel C after transfection into MDCK cells. Bars, 5 μm.
Similar articles
- The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium.
Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, Rodriguez-Boulan E. Deora AA, et al. Mol Biol Cell. 2004 Sep;15(9):4148-65. doi: 10.1091/mbc.e04-01-0058. Epub 2004 Jun 23. Mol Biol Cell. 2004. PMID: 15215314 Free PMC article. - Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells.
Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. Roush DL, et al. J Biol Chem. 1998 Oct 9;273(41):26862-9. doi: 10.1074/jbc.273.41.26862. J Biol Chem. 1998. PMID: 9756932 - ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor.
Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adélaïde J, Margolis B, Birnbaum D. Borg JP, et al. Nat Cell Biol. 2000 Jul;2(7):407-14. doi: 10.1038/35017038. Nat Cell Biol. 2000. PMID: 10878805 - Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants.
Dempsey PJ, Meise KS, Coffey RJ. Dempsey PJ, et al. Exp Cell Res. 2003 May 1;285(2):159-74. doi: 10.1016/s0014-4827(03)00035-1. Exp Cell Res. 2003. PMID: 12706112 - Sequence and structural insights of monoleucine-based sorting motifs contained within the cytoplasmic domains of basolateral proteins.
Harmych SJ, Tydings CW, Meiler J, Singh B. Harmych SJ, et al. Front Cell Dev Biol. 2024 Mar 1;12:1379224. doi: 10.3389/fcell.2024.1379224. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38495621 Free PMC article. Review.
Cited by
- Loss of EGF receptor polarity enables homeostatic imbalance in epithelial-cell models.
Carlin CR, Ngalula S. Carlin CR, et al. Mol Biol Cell. 2023 Nov 1;34(12):ar116. doi: 10.1091/mbc.E23-04-0133. Epub 2023 Aug 30. Mol Biol Cell. 2023. PMID: 37647145 Free PMC article. - Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells.
Singh B, Coffey RJ. Singh B, et al. Annu Rev Physiol. 2014;76:275-300. doi: 10.1146/annurev-physiol-021113-170406. Epub 2013 Nov 8. Annu Rev Physiol. 2014. PMID: 24215440 Free PMC article. Review. - Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.
Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ. Castorino JJ, et al. Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article. - Scaffold Proteins Regulating Extracellular Regulated Kinase Function in Cardiac Hypertrophy and Disease.
Liang Y, Sheikh F. Liang Y, et al. Front Pharmacol. 2016 Feb 29;7:37. doi: 10.3389/fphar.2016.00037. eCollection 2016. Front Pharmacol. 2016. PMID: 26973524 Free PMC article. Review. - Polarized trafficking of the sorting receptor SorLA in neurons and MDCK cells.
Klinger SC, Højland A, Jain S, Kjolby M, Madsen P, Svendsen AD, Olivecrona G, Bonifacino JS, Nielsen MS. Klinger SC, et al. FEBS J. 2016 Jul;283(13):2476-93. doi: 10.1111/febs.13758. Epub 2016 Jun 6. FEBS J. 2016. PMID: 27192064 Free PMC article.
References
- Alroy, I., and Y. Yarden. 1997. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 410:83-86. - PubMed
- Borg, J. P., S. Marchetto, A. Le Bivic, V. Ollendorff, F. Jaulin-Bastard, H. Saito, E. Fournier, J. Adelaide, B. Margolis, and D. Birnbaum. 2000. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat. Cell Biol. 2:407-414. - PubMed
- Breuza, L., M. Garcia, M. H. Delgrossi, and A. Le Bivic. 2002. Role of the membrane-proximal O-glycosylation site in sorting of the human receptor for neurotrophins to the apical membrane of MDCK cells. Exp. Cell Res. 273:178-186. - PubMed
- Carraway, K. L., III, and C. Sweeney. 2001. Localization and modulation of ErbB receptor tyrosine kinases. Curr. Opin. Cell Biol. 13:125-130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous