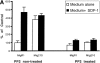Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells - PubMed (original) (raw)
Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells
Andrzej Ptasznik et al. J Exp Med. 2002.
Abstract
Stromal-derived factor (SDF)-1 and its G protein-coupled receptor, CXCR4, regulate stem/progenitor cell migration and retention in the marrow and are required for hematopoiesis. We show here an interaction between CXCR4 and the Src-related kinase, Lyn, in normal progenitors. We demonstrate that CXCR4-dependent stimulation of Lyn is associated with the activation of phosphatidylinositol 3-kinase (PI3-kinase). This chemokine signaling, which involves a Src-related kinase and PI3-kinase, appears to be a target for BCR/ABL, a fusion oncoprotein expressed only in leukemia cells. We show that the binding of phosphorylated BCR/ABL to Lyn results in the constitutive activation of Lyn and PI3-kinase, along with a total loss of responsiveness of these kinases to SDF-1 stimulation. Inhibition of BCR/ABL tyrosine kinase with STI571 restores Lyn responsiveness to SDF-1 signaling. Thus, BCR/ABL perturbs Lyn function through a tyrosine kinase-dependent mechanism. Accordingly, the blockade of Lyn tyrosine kinase inhibits both BCR/ABL-dependent and CXCR4-dependent cell movements. Our results demonstrate, for the first time, that Lyn-mediated pathological crosstalk exists between BCR/ABL and the CXCR4 pathway in leukemia cells, which disrupts chemokine signaling and chemotaxis, and increases the ability of immature cells to escape from the marrow. These results define a Src tyrosine kinases-dependent mechanism whereby BCR/ABL (and potentially other oncoproteins) dysregulates G protein-coupled receptor signaling and function of mammalian precursors.
Figures
Figure 1.
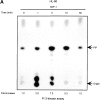
SDF-1 stimulates Lyn kinase activity in hematopoietic cells. (A) Lyn autophosphorylation is increased after treatment of HL-60 cells with SDF-1; SDF-1 induces an increase in Lyn phosphorylation of substrate enolase. A similar time course was obtained using SCF as a positive control. (B) Lyn and enolase phosphorylation are stimulated after treatment of CD34+ marrow progenitors with SDF-1. Cells in all experiments were stimulated for the indicated times with either SDF-1 or SCF, lysed, and immunoprecipitated with anti-Lyn antibodies. Immune complex in vitro kinase assays were performed as described in Materials and Methods. The changes in Lyn autophosphorylation are indicated (by fold increase) based on the densitometry. The results shown are representative of two experiments.
Figure 1.
SDF-1 stimulates Lyn kinase activity in hematopoietic cells. (A) Lyn autophosphorylation is increased after treatment of HL-60 cells with SDF-1; SDF-1 induces an increase in Lyn phosphorylation of substrate enolase. A similar time course was obtained using SCF as a positive control. (B) Lyn and enolase phosphorylation are stimulated after treatment of CD34+ marrow progenitors with SDF-1. Cells in all experiments were stimulated for the indicated times with either SDF-1 or SCF, lysed, and immunoprecipitated with anti-Lyn antibodies. Immune complex in vitro kinase assays were performed as described in Materials and Methods. The changes in Lyn autophosphorylation are indicated (by fold increase) based on the densitometry. The results shown are representative of two experiments.
Figure 2.
SDF-1 stimulates PI3-kinase activity in hematopoietic cells. (A) Cellular p85 subunit-dependent PI3-kinase activity is increased after SDF-1 treatment of HL-60 cells. (B) Cellular p85 subunit-dependent PI3-kinase activity is stimulated after SDF-1 treatment of CD34+ bone marrow progenitors. (C) Lyn-associated PI3-kinase activity is increased in SDF-1–stimulated HL-60 cells. Cells were stimulated for the indicated times with SDF-1, lysed, and immunoprecipitated with anti-PI3-kinase p85 antibodies (A, B, and C-a 1), anti-Lyn antibodies (2C-a, 2-5, and 2C-b), or control anti-Lck antibodies. PI3-kinase activity was measured in immunoprecipitates as described in Materials and Methods. The indicated changes in PIP are based on densitometry values. Aliquots of total cell lysates were subjected to either Lyn immunoprecipitation and immunoblotting with anti-p85PI3K Ab or immunoblotting with anti-Lyn ab Similar amounts of Lyn protein were present in stimulated and control cells (C-b). The results shown are representative of two experiments.
Figure 2.
SDF-1 stimulates PI3-kinase activity in hematopoietic cells. (A) Cellular p85 subunit-dependent PI3-kinase activity is increased after SDF-1 treatment of HL-60 cells. (B) Cellular p85 subunit-dependent PI3-kinase activity is stimulated after SDF-1 treatment of CD34+ bone marrow progenitors. (C) Lyn-associated PI3-kinase activity is increased in SDF-1–stimulated HL-60 cells. Cells were stimulated for the indicated times with SDF-1, lysed, and immunoprecipitated with anti-PI3-kinase p85 antibodies (A, B, and C-a 1), anti-Lyn antibodies (2C-a, 2-5, and 2C-b), or control anti-Lck antibodies. PI3-kinase activity was measured in immunoprecipitates as described in Materials and Methods. The indicated changes in PIP are based on densitometry values. Aliquots of total cell lysates were subjected to either Lyn immunoprecipitation and immunoblotting with anti-p85PI3K Ab or immunoblotting with anti-Lyn ab Similar amounts of Lyn protein were present in stimulated and control cells (C-b). The results shown are representative of two experiments.
Figure 2.
SDF-1 stimulates PI3-kinase activity in hematopoietic cells. (A) Cellular p85 subunit-dependent PI3-kinase activity is increased after SDF-1 treatment of HL-60 cells. (B) Cellular p85 subunit-dependent PI3-kinase activity is stimulated after SDF-1 treatment of CD34+ bone marrow progenitors. (C) Lyn-associated PI3-kinase activity is increased in SDF-1–stimulated HL-60 cells. Cells were stimulated for the indicated times with SDF-1, lysed, and immunoprecipitated with anti-PI3-kinase p85 antibodies (A, B, and C-a 1), anti-Lyn antibodies (2C-a, 2-5, and 2C-b), or control anti-Lck antibodies. PI3-kinase activity was measured in immunoprecipitates as described in Materials and Methods. The indicated changes in PIP are based on densitometry values. Aliquots of total cell lysates were subjected to either Lyn immunoprecipitation and immunoblotting with anti-p85PI3K Ab or immunoblotting with anti-Lyn ab Similar amounts of Lyn protein were present in stimulated and control cells (C-b). The results shown are representative of two experiments.
Figure 2.
SDF-1 stimulates PI3-kinase activity in hematopoietic cells. (A) Cellular p85 subunit-dependent PI3-kinase activity is increased after SDF-1 treatment of HL-60 cells. (B) Cellular p85 subunit-dependent PI3-kinase activity is stimulated after SDF-1 treatment of CD34+ bone marrow progenitors. (C) Lyn-associated PI3-kinase activity is increased in SDF-1–stimulated HL-60 cells. Cells were stimulated for the indicated times with SDF-1, lysed, and immunoprecipitated with anti-PI3-kinase p85 antibodies (A, B, and C-a 1), anti-Lyn antibodies (2C-a, 2-5, and 2C-b), or control anti-Lck antibodies. PI3-kinase activity was measured in immunoprecipitates as described in Materials and Methods. The indicated changes in PIP are based on densitometry values. Aliquots of total cell lysates were subjected to either Lyn immunoprecipitation and immunoblotting with anti-p85PI3K Ab or immunoblotting with anti-Lyn ab Similar amounts of Lyn protein were present in stimulated and control cells (C-b). The results shown are representative of two experiments.
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 3.
Defect of SDF-1–induced Lyn/PI3-kinase pathway in chronic myeloid leukemia. (A and B) Representative time course of Lyn and p85-dependent PI3-kinase phosphorylation in SDF-1–stimulated BCR/ABL-positive leukemic blasts (CML). Lyn tyrosine kinase activity (A) and PI3-kinase activity (B) are not increased after SDF-1 stimulation. This is in contrast to time courses observed in normal CD34+ cells and BCR/ABL-negative HL-60 cells, which are presented in Fig. 1, 2, and 3, E and F. Results are representative of four experiments. (C) Expression of exogenous BCR/ABL in HL-60 cells. Infected cells were analyzed for protein levels of exogenous BCR/ABL by Western blot and for BCR/ABL tyrosine kinase activity by immune complex in vitro kinase assays, as described in Materials and Methods. P56/53-kD tyrosine phosphorylated protein is detectable in BCR/ABL precipitates from BCR/ABL-infected Mig210 cells, but not from cells infected with control vector (MigR1). (D) Expression of endogenous Lyn in BCR/ABL-infected HL-60 cells. P210-kD tyrosine phosphorylated protein is coimmunoprecipitated with anti-Lyn antibody in Mig210, but not MigR1 cells. This protein comigrates with endogenous BCR/ABL in K562 cells (positive control). The phosphorylated 210-kD protein was identified as BCR/ABL by secondary p210 BCR/ABL immunoprecipitations on primary Lyn precipitates. The secondary IPs were performed after the disruption of protein complexes by disruption buffer (see Material and Methods). The position of BCR/ABL is indicated by the arrow. The relatively weak signal from secondary IPs is explained by the loss of precipitated proteins during disruption and reprecipitation. Results are representative of two experiments (D-a). (E and F) Comparison of SDF-1–stimulated Lyn and PI3-kinase activities and protein levels in BCR/ABL-negative HL-60 cells and BCR/ABL-positive HL-60 cells. Lyn autophosphorylation is inducible by SDF-1 in MigR1, but not in Mig210 cells (E). P85 subunit-dependent PI3-kinase activity is stimulated by SDF-1 in control cells, but not in Mig210 cells (F).
Figure 4.
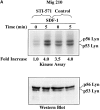
Effect of STI-571 on SDF-1–regulated Lyn/PI3-kinase pathway in BCR/ABL-positive cells. (A) Comparison of SDF-1–stimulated Lyn activity and protein levels in BCR/ABL-positive HL-60 cells exposed or not exposed to 1.0 μM STI-571. (B) Comparison of SDF-1–stimulated PI3-kinase activity and protein levels in BCR/ABL-positive HL-60 cells exposed or not exposed to 1.0 μM STI-571. The changes in Lyn or PI3-kinase phosphorylation are indicated (by fold increase) based on the densitometry.
Figure 4.
Effect of STI-571 on SDF-1–regulated Lyn/PI3-kinase pathway in BCR/ABL-positive cells. (A) Comparison of SDF-1–stimulated Lyn activity and protein levels in BCR/ABL-positive HL-60 cells exposed or not exposed to 1.0 μM STI-571. (B) Comparison of SDF-1–stimulated PI3-kinase activity and protein levels in BCR/ABL-positive HL-60 cells exposed or not exposed to 1.0 μM STI-571. The changes in Lyn or PI3-kinase phosphorylation are indicated (by fold increase) based on the densitometry.
Figure 5.
Effect of PP2 on CXCR4-dependent and BCR/ABL-dependent cell movements. Inhibition of CXCR4-dependent cell movements in Lyn-deficient primary cells. (A) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined as described under Materials and Methods and Results in BCR/ABL-negative HL-60 cells (MigR1) and BCR/ABL-positive HL-60 cells (Mig210). The initial number of BCR/ABL-negative MigR1 cells that migrated to medium alone was set to 100%. The results shown (mean ± SD) are averages of six experiments. (B) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined in hematopoietic precursor cells Mo7e as described under Materials and Methods and Results. The results shown are representative of six experiments. (C) The suppression of CXCR4-dependent chemotaxis by PP2, but not by its inactive analogue PP3, in CD34+ human primary myeloid cells. PMA-induced migration is normal in PP2-pretreated CD34+ cells. Treatment and migration were performed as described in Materials and Methods. The results shown represent the average ± range of four separate determinations with different normal cell donors. (D) The reduction of SDF-1-induced migration in Lyn-deficient mononuclear bone marrow cells from knock-out mice. PMA-induced migration appears to be normal in these cells. Data represents the mean and standard deviation of eight samples with eight different animals. Treatment with factors and migration assays were performed as described in Materials and Methods.
Figure 5.
Effect of PP2 on CXCR4-dependent and BCR/ABL-dependent cell movements. Inhibition of CXCR4-dependent cell movements in Lyn-deficient primary cells. (A) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined as described under Materials and Methods and Results in BCR/ABL-negative HL-60 cells (MigR1) and BCR/ABL-positive HL-60 cells (Mig210). The initial number of BCR/ABL-negative MigR1 cells that migrated to medium alone was set to 100%. The results shown (mean ± SD) are averages of six experiments. (B) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined in hematopoietic precursor cells Mo7e as described under Materials and Methods and Results. The results shown are representative of six experiments. (C) The suppression of CXCR4-dependent chemotaxis by PP2, but not by its inactive analogue PP3, in CD34+ human primary myeloid cells. PMA-induced migration is normal in PP2-pretreated CD34+ cells. Treatment and migration were performed as described in Materials and Methods. The results shown represent the average ± range of four separate determinations with different normal cell donors. (D) The reduction of SDF-1-induced migration in Lyn-deficient mononuclear bone marrow cells from knock-out mice. PMA-induced migration appears to be normal in these cells. Data represents the mean and standard deviation of eight samples with eight different animals. Treatment with factors and migration assays were performed as described in Materials and Methods.
Figure 5.
Effect of PP2 on CXCR4-dependent and BCR/ABL-dependent cell movements. Inhibition of CXCR4-dependent cell movements in Lyn-deficient primary cells. (A) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined as described under Materials and Methods and Results in BCR/ABL-negative HL-60 cells (MigR1) and BCR/ABL-positive HL-60 cells (Mig210). The initial number of BCR/ABL-negative MigR1 cells that migrated to medium alone was set to 100%. The results shown (mean ± SD) are averages of six experiments. (B) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined in hematopoietic precursor cells Mo7e as described under Materials and Methods and Results. The results shown are representative of six experiments. (C) The suppression of CXCR4-dependent chemotaxis by PP2, but not by its inactive analogue PP3, in CD34+ human primary myeloid cells. PMA-induced migration is normal in PP2-pretreated CD34+ cells. Treatment and migration were performed as described in Materials and Methods. The results shown represent the average ± range of four separate determinations with different normal cell donors. (D) The reduction of SDF-1-induced migration in Lyn-deficient mononuclear bone marrow cells from knock-out mice. PMA-induced migration appears to be normal in these cells. Data represents the mean and standard deviation of eight samples with eight different animals. Treatment with factors and migration assays were performed as described in Materials and Methods.
Figure 5.
Effect of PP2 on CXCR4-dependent and BCR/ABL-dependent cell movements. Inhibition of CXCR4-dependent cell movements in Lyn-deficient primary cells. (A) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined as described under Materials and Methods and Results in BCR/ABL-negative HL-60 cells (MigR1) and BCR/ABL-positive HL-60 cells (Mig210). The initial number of BCR/ABL-negative MigR1 cells that migrated to medium alone was set to 100%. The results shown (mean ± SD) are averages of six experiments. (B) The inhibition of SDF-1–regulated chemotaxis by PP2 was determined in hematopoietic precursor cells Mo7e as described under Materials and Methods and Results. The results shown are representative of six experiments. (C) The suppression of CXCR4-dependent chemotaxis by PP2, but not by its inactive analogue PP3, in CD34+ human primary myeloid cells. PMA-induced migration is normal in PP2-pretreated CD34+ cells. Treatment and migration were performed as described in Materials and Methods. The results shown represent the average ± range of four separate determinations with different normal cell donors. (D) The reduction of SDF-1-induced migration in Lyn-deficient mononuclear bone marrow cells from knock-out mice. PMA-induced migration appears to be normal in these cells. Data represents the mean and standard deviation of eight samples with eight different animals. Treatment with factors and migration assays were performed as described in Materials and Methods.
Figure 6.
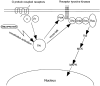
Proposed model for interaction between oncoproteins and G protein-coupled signal transduction pathways in transformed mammalian cells. BCR/ABL oncoprotein interferes with the Src-related kinase Lyn signaling, which is regulated by G protein–coupled chemokine receptors in hematopoietic cells. A previously defined direct interaction of G protein subunits with Src family kinases (references and 27) and linkage of G protein–coupled receptor via Src kinases to the receptor tyrosine kinase complex and its downstream signaling cascades (references – and 44) is indicated, as described in the text. The signaling paradigm we have depicted provides a mechanism to explain the ability of BCR/ABL, and potentially other oncoproteins, to couple simultaneously to multiple signaling transducers (e.g., PI3-kinase, Shc adaptor protein, receptor tyrosine kinase, and the RAS-to-MAPK cascade). This paradigm may also provide further insight into the remarkable ability of Src family kinases to transform various cell types.
Similar articles
- Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts.
Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, Cortes J, Andreeff M, Konopleva M. Tabe Y, et al. Leukemia. 2012 May;26(5):883-92. doi: 10.1038/leu.2011.291. Epub 2011 Oct 18. Leukemia. 2012. PMID: 22005789 - BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells.
Chen YY, Malik M, Tomkowicz BE, Collman RG, Ptasznik A. Chen YY, et al. Blood. 2008 May 15;111(10):5182-6. doi: 10.1182/blood-2007-10-117705. Epub 2008 Mar 13. Blood. 2008. PMID: 18339898 Free PMC article. - p210BCR-ABL inhibits SDF-1 chemotactic response via alteration of CXCR4 signaling and down-regulation of CXCR4 expression.
Geay JF, Buet D, Zhang Y, Foudi A, Jarrier P, Berthebaud M, Turhan AG, Vainchenker W, Louache F. Geay JF, et al. Cancer Res. 2005 Apr 1;65(7):2676-83. doi: 10.1158/0008-5472.CAN-04-2152. Cancer Res. 2005. PMID: 15805265 - Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells.
Wu J, Meng F, Lu H, Kong L, Bornmann W, Peng Z, Talpaz M, Donato NJ. Wu J, et al. Blood. 2008 Apr 1;111(7):3821-9. doi: 10.1182/blood-2007-08-109330. Epub 2008 Jan 30. Blood. 2008. PMID: 18235045 Free PMC article. - Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib.
Dillmann F, Veldwijk MR, Laufs S, Sperandio M, Calandra G, Wenz F, Zeller J, Fruehauf S. Dillmann F, et al. Leuk Lymphoma. 2009 Oct;50(10):1676-86. doi: 10.1080/10428190903150847. Leuk Lymphoma. 2009. PMID: 19657955
Cited by
- Essential Role of Lyn in Fibrosis.
Pham H, Birtolo C, Chheda C, Yang W, Rodriguez MD, Liu ST, Gugliotta G, Lewis MS, Cirulli V, Pandol SJ, Ptasznik A. Pham H, et al. Front Physiol. 2016 Aug 31;7:387. doi: 10.3389/fphys.2016.00387. eCollection 2016. Front Physiol. 2016. PMID: 27630579 Free PMC article. - Dasatinib inhibits CXCR4 signaling in chronic lymphocytic leukaemia cells and impairs migration towards CXCL12.
McCaig AM, Cosimo E, Leach MT, Michie AM. McCaig AM, et al. PLoS One. 2012;7(11):e48929. doi: 10.1371/journal.pone.0048929. Epub 2012 Nov 2. PLoS One. 2012. PMID: 23133664 Free PMC article. - CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptor.
Dar WA, Knechtle SJ. Dar WA, et al. Immunology. 2007 Apr;120(4):467-85. doi: 10.1111/j.1365-2567.2006.02534.x. Epub 2007 Jan 22. Immunology. 2007. PMID: 17250586 Free PMC article. - Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1 alpha through Lyn kinase.
Malik M, Chen YY, Kienzle MF, Tomkowicz BE, Collman RG, Ptasznik A. Malik M, et al. J Immunol. 2008 Oct 1;181(7):4632-7. doi: 10.4049/jimmunol.181.7.4632. J Immunol. 2008. PMID: 18802065 Free PMC article. - Neutrophil migration: moving from zebrafish models to human autoimmunity.
Shelef MA, Tauzin S, Huttenlocher A. Shelef MA, et al. Immunol Rev. 2013 Nov;256(1):269-81. doi: 10.1111/imr.12124. Immunol Rev. 2013. PMID: 24117827 Free PMC article. Review.
References
- Groffen, J., J.R. Stephenson, N. Heisterkamp, A. de Klein, C.R. Bartram, and G. Grosveld. 1984. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 36:93–99. - PubMed
- Daley, G.Q., and N.Y. Ben. 1991. Implicating the bcr/abl gene in the pathogenesis of Philadelphia chromosome-positive human leukemia. Adv. Cancer Res. 57:151–184. - PubMed
- Kabarowski, J.H.S., and O.N. Witte. 2000. Consequences of BCR-ABL expression within the hematopoietic stem cell in chronic myeloid leukemia. Stem Cells. 18:399–408. - PubMed
- Salgia, R., J.-L. Li, S.H. Lo, B. Brunckhorst, G.S. Kansas, S. Sobhany, Y. Sun, E. Pisick, M. Hallek, T. Ernst, et al. 1995. Molecular cloning of human paxillin, a focal adhesion protein phosphorylated by p210Bcr/ABL. J. Biol. Chem. 270:5039–5047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous