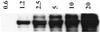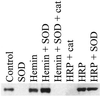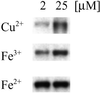Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2- reaction - PubMed (original) (raw)
Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2- reaction
Douglas D Thomas et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The chemical origins of nitrated tyrosine residues (NT) formed in proteins during a variety of pathophysiological conditions remain controversial. Although numerous studies have concluded that NT is a signature for peroxynitrite (ONOO(-)) formation, other works suggest the primary involvement of peroxidases. Because metal homeostasis is often disrupted in conditions bearing NT, the role of metals as catalysts for protein nitration was examined. Cogeneration of nitric oxide (NO) and superoxide (O(2)(-)), from spermine/NO (2.7 microM/min) and xanthine oxidase (1-28 microM O(2)(-)/min), respectively, resulted in protein nitration only when these species were produced at approximately equivalent rates. Addition of ferriprotoporphyrin IX (hemin) to this system increased nitration over a broad range of O(2)(-) concentrations with respect to NO. Nitration in the presence of superoxide dismutase but not catalase suggested that ONOO(-) might not be obligatory to this process. Hemin-mediated NT formation required only the presence of NO(2)(-) and H(2)O(2), which are stable end-products of NO and O(2)(-) degradation. Ferrous, ferric, and cupric ions were also effective catalysts, indicating that nitration is mediated by species capable of Fenton-type chemistry. Although ONOO(-) can nitrate proteins, there are severe spatial and temporal constraints on this reaction. In contrast, accumulation of metals and NO(2)(-) subsequent to NO synthase activity can result in far less discriminate nitration in the presence of an H(2)O(2) source. Metal catalyzed nitration may account for the observed specificity of protein nitration seen under pathological conditions, suggesting a major role for translocated metals and the labilization of heme in NT formation.
Figures
Figure 1
Immunoblot demonstrating nitration of BSA by bolus ONOO−. Synthetic ONOO− (0.6–20 μM) was added to 1 ml of PBS containing DPTA (50 μM) and BSA (80 μg/ml), followed by 30 min incubation at 37°C. BSA (1.4 μg) was loaded to each lane and subjected to PAGE on 10% Tris-glycine acrylamide gels. Bands, representing BSA (66 kDa), were probed with rabbit polyclonal 3-nitrotyrosine antibodies (0.5 μg/ml) and HRP-conjugated secondary antibodies (1:10,000).
Figure 2
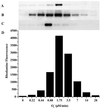
Oxidative and nitrative profile elicited by co-generation of NO and O ± hemin or Fe2+. SPER/NO (100 μM; 2.7 μM NO/min), HX (500 μM), XO (O
fluxes of 0.22–28 μM/min), and BSA (80 μg/ml) or DHR (50 μM) were added to 200 μl of PBS containing DTPA (50 μM) in a black-walled microtiter plate. After a 1.5-h incubation at 37°C, NT Western blot analysis (A_–_C) or rhodamine formation (D; λex/em 500/570) was measured. A and D, SPER/NO + XO; B, SPER/NO + XO + hemin (1 μM); C, SPER/NO + XO + Fe2+ (5 μM, without DTPA).
Figure 3
Immunoblot demonstrating nitration of BSA by co-generation of NO/O under various conditions. SPER/NO (100 μM), HX (500 μM), XO (O
fluxes of 1.75 μM/min), and BSA (80 μg/ml) were added to 200 μl of PBS containing DTPA (50 μM) in a black-walled microtiter plate. SPER/NO and XO concentrations were chosen that corresponded to maximal DHR oxidation (Fig. 2_D_) indicating optimal ONOO− formation. In addition, each well contained SOD (3.5 μg/ml), hemin (2 μM), catalase (100 units/ml), or HRP (100 nM, 1 unit/ml), as indicated. After a 1.5-h incubation at 37°C, PAGE (1.4 μg of BSA) and Western blot analysis were preformed. The bands represent BSA (66 kDa) run on a single gel with control lanes not shown.
Figure 4
Immuno dot-blot demonstrating nitration of BSA from NO/H2O2/hemin. BSA (80 μg/ml), NaNO2 (1–100 μM), H2O2 (1–100 μM), and hemin (0.25–4 μM) were added to 1 ml of PBS containing DTPA (50 μM), followed by a 1.5-h incubation at 37°C. Each dot represents 14 μg of protein vacuum transferred to a PDVF membrane and probed with rabbit polyclonal 3-nitrotyrosine antibodies (0.5 μg/ml) and HRP-conjugated secondary antibodies (1:10,000). A and B represent separate membranes and therefore intensity differences between them should not be directly compared.
Figure 5
Immunoblot demonstrating nitration of Aβ ± BSA and hemin. Aβ (80 μg/ml), NaNO2 (100 μM), and H2O2 (100 μM) were added to 1 ml of PBS containing DTPA (50 μM) and hemin (1 or 0.5 μM) and BSA (80 μg/ml), as indicated. After a 1.5-h incubation at 37°C each sample was subjected to PAGE on 4–20% Tris-glycine acrylamide gel followed by membrane transfer and was probed with rabbit polyclonal 3-nitrotyrosine antibodies (0.5 μg/ml) and HRP-conjugated secondary antibodies (1:10,000).
Figure 6
Immuno dot-blot demonstrating nitration of cellular proteins by hemin, H2O2, and SPER/NO. MCF-7 cells in culture (90% confluent) were exposed for 2 h to 100 μM H2O2 and 100 μM SPER/NO in serum-free media. Cells were either pretreated (for 1 h) with hemin (4–32 μM) and washed five time, or cotreated with hemin. Cell proteins were extracted, vacuum transferred (14 μg per dot) to a PDVF membrane, and probed with rabbit polyclonal 3-nitrotyrosine antibodies (0.5 μg/ml) and HRP-conjugated secondary antibodies (1:10,000).
Figure 7
Immunoblot demonstrating nitration of BSA by free metals. CuCl2, FeCl3, or FeSO4 at 2 or 25 μM was added to 1 ml of BSA solution (80 μg/ml PBS) containing NaNO2 (100 μM) + H2O2 (100 μM). After a 2-h incubation at 37°C, each sample (1.4 μg of BSA per lane) was subjected to PAGE on 10% Tris-glycine acrylamide gels followed by membrane transfer and was probed with rabbit polyclonal 3-nitrotyrosine antibodies (0.5 μg/ml) and HRP-conjugated secondary antibodies (1:10,000). Bands represent BSA (66 kDa) run on a single gel.
Similar articles
- Hemin-H2O2-NO2(-) induced protein oxidation and tyrosine nitration are different from those of SIN-1: a study on glutamate dehydrogenase nitrative/oxidative modification.
Zhang Y, Lu N, Gao Z. Zhang Y, et al. Int J Biochem Cell Biol. 2009 Apr;41(4):907-15. doi: 10.1016/j.biocel.2008.08.040. Epub 2008 Sep 11. Int J Biochem Cell Biol. 2009. PMID: 18835362 - Metal-catalyzed protein tyrosine nitration in biological systems.
Campolo N, Bartesaghi S, Radi R. Campolo N, et al. Redox Rep. 2014 Nov;19(6):221-31. doi: 10.1179/1351000214Y.0000000099. Epub 2014 Jun 30. Redox Rep. 2014. PMID: 24977336 Free PMC article. Review. - Tyrosine nitration by superoxide and nitric oxide fluxes in biological systems: modeling the impact of superoxide dismutase and nitric oxide diffusion.
Quijano C, Romero N, Radi R. Quijano C, et al. Free Radic Biol Med. 2005 Sep 15;39(6):728-41. doi: 10.1016/j.freeradbiomed.2005.04.014. Free Radic Biol Med. 2005. PMID: 16109303 - Completely different effects of desferrioxamine on hemin/nitrite/H2O2-induced bovine serum albumin nitration and oxidation.
Lu N, Zhang M, Li H, Gao Z. Lu N, et al. Chem Res Toxicol. 2008 Jun;21(6):1229-34. doi: 10.1021/tx800013e. Epub 2008 May 7. Chem Res Toxicol. 2008. PMID: 18459802 - Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide.
Squadrito GL, Pryor WA. Squadrito GL, et al. Free Radic Biol Med. 1998 Sep;25(4-5):392-403. doi: 10.1016/s0891-5849(98)00095-1. Free Radic Biol Med. 1998. PMID: 9741578 Review.
Cited by
- Differential effects of reactive nitrogen species on DNA base excision repair initiated by the alkyladenine DNA glycosylase.
Jones LE Jr, Ying L, Hofseth AB, Jelezcova E, Sobol RW, Ambs S, Harris CC, Espey MG, Hofseth LJ, Wyatt MD. Jones LE Jr, et al. Carcinogenesis. 2009 Dec;30(12):2123-9. doi: 10.1093/carcin/bgp256. Carcinogenesis. 2009. PMID: 19864471 Free PMC article. - Mitigation of peroxynitrite-mediated nitric oxide (NO) toxicity as a mechanism of induced adaptive NO resistance in the CNS.
Bishop A, Gooch R, Eguchi A, Jeffrey S, Smallwood L, Anderson J, Estevez AG. Bishop A, et al. J Neurochem. 2009 Apr;109(1):74-84. doi: 10.1111/j.1471-4159.2009.05884.x. Epub 2009 Jan 13. J Neurochem. 2009. PMID: 19183270 Free PMC article. - Bioanalytical profile of the L-arginine/nitric oxide pathway and its evaluation by capillary electrophoresis.
Boudko DY. Boudko DY. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 May 15;851(1-2):186-210. doi: 10.1016/j.jchromb.2007.02.011. Epub 2007 Feb 15. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17329176 Free PMC article. Review. - NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria.
Gusarov I, Nudler E. Gusarov I, et al. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13855-60. doi: 10.1073/pnas.0504307102. Epub 2005 Sep 19. Proc Natl Acad Sci U S A. 2005. PMID: 16172391 Free PMC article. - 4-Hydr-oxy-3-meth-oxy-5-nitro-aceto-phenone (5-nitro-apocynin).
Babu S, Raghavamenon AC, Fronczek FR, Uppu RM. Babu S, et al. Acta Crystallogr Sect E Struct Rep Online. 2009 Aug 29;65(Pt 9):o2292-3. doi: 10.1107/S160053680903390X. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21577684 Free PMC article.
References
- Wattanapitayakul S K, Weinstein D M, Holycross B J, Bauer J A. FASEB J. 2000;14:271–278. - PubMed
- Ferdinandy P, Danial H, Ambrus I, Rothery R A, Schulz R. Circ Res. 2000;87:241–247. - PubMed
- Kondo S, Toyokuni S, Tsuruyama T, Ozeki M, Tachibana T, Echizenya M, Hiai H, Onodera H, Imamura M. Cancer Lett. 2002;179:87–93. - PubMed
- Mendes R V, Martins A R, de Nucci G, Murad F, Soares F A. Histopathology. 2001;39:172–178. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials