p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene - PubMed (original) (raw)
p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene
Shanthi Adimoolam et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The p53 tumor suppressor gene product is a transcription factor involved in cell-cycle regulation, apoptosis, and DNA repair. We and others have shown that p53 is required for efficient nucleotide excision repair (NER) of UV-induced DNA lesions. p53-deficient cells are defective in the repair of UV photoproducts in genomic DNA but proficient for transcription-coupled repair. Therefore, we examined whether p53 regulates the expression of genes required for global genomic repair. In this study, we demonstrate that the mRNA and protein products of the xeroderma pigmentosum group C (XPC) gene are UV-inducible in a time- and dose-dependent manner in human WI38 fibroblasts and HCT116 colorectal cancer cells wild type for p53. However, no significant induction of XPC was observed in p53-deficient counterparts to these cells. Furthermore, regulated expression of wild-type p53 in p53 null Li-Fraumeni syndrome human fibroblasts significantly augmented the expression of XPC protein. Analysis of the human XPC gene sequence revealed a putative p53 response element in the XPC promoter that was capable of mediating sequence-specific DNA binding to p53 in vitro. These results provide strong evidence that the NER gene XPC is a DNA damage-inducible and p53-regulated gene and likely plays a role in the p53-dependent NER pathway.
Figures
Figure 1
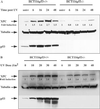
XPC and p53 expression in HCT116p53+/+ and HCT116p53−/− cells. (A) HCT116 wt p53 cells as well as cells with homozygous disruptions of the p53 gene were treated with 15 J/m2 UV and lysed at the indicated times thereafter. Total protein was extracted and quantitated as described in Experimental Procedures. Anti-XPC and anti-p53 mouse monoclonal antibodies were used to evaluate XPC and p53 levels after UV irradiation. Tubulin (Sigma) was used as a loading control. unirr, unirradiated. (B) The cells were treated with the varying doses of UV indicated and harvested 24 h later. Antibodies used were as described above. Fold inductions at the various times post-UV were quantified relative to the respective unirradiated levels by using QUANTITY ONE software (Bio-Rad).
Figure 2
XPC and p53 expression in WI38 and WI38-E6 cells. (A) Expression of XPC and p53 was evaluated in WI38 normal human fibroblasts as well as in the E6-transformed p53 null counterpart. The times indicated are incubation times after treatment with 15 J/m2 UV irradiation. Antibodies used are as described above. unirr, unirradiated. (B) UV dose response of p53 and XPC expression was studied by using WI38 and WI38-E6 cells. Cells were harvested 48 h after UV treatment. Fold inductions were calculated as described in the Fig. 1 legend.
Figure 3
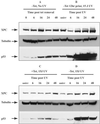
XPC and p53 levels in Li–Fraumeni p53−/− cells containing a tet-regulatable human p53 cDNA. Levels of XPC and p53 proteins were examined after reintroduction of p53 into a p53 mutant background by using the p53-tet regulatable cell line. (A) Tet was withdrawn from the medium, and the cells were sampled at the times indicated after tet removal. Cells were not treated with UV irradiation (unirr). (B) Tet was removed from the medium 12 h before UV irradiation and treated with 15 J/m2. The times indicated are incubation times post-UV irradiation. (C) Medium was supplemented with 2 μg/ml tet, and cells were irradiated with 15 J/m2 UV. The times indicated are post-UV irradiation. (D) Tet was removed at the time of UV irradiation. The times indicated are times following UV and tet withdrawal. The antibodies used were as described in the Fig. 1 legend.
Figure 4
Northern blot analysis of XPC mRNA expression in HCT116p53+/+, p53−/−, and WI38 and WI38-E6 cells. (A) Analysis of XPC mRNA expression in HCT116 cells. Times indicated are incubation times after 15 J/m2 UV irradiation. XPC and GAPDH riboprobes were generated as described in Experimental Procedures. GAPDH was probed to control for loading. Fold inductions at the various times post-UV were quantified relative to the respective unirradiated (unirr) levels by using phosphorimager densitometry and QUANTITY ONE software (Bio-Rad). (B) Northern blot of XPC levels in WI38 and WI38-E6 cells. The procedure used was as described in A.
Figure 5
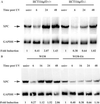
Electrophoretic mobility-shift assay illustrates binding of p53 to the XPC gene p53 response element in vitro. (A) Shown is a schematic (not drawn to scale) of a portion of the promoter and exon 1 of the XPC gene. The numbers represented indicate the nucleotide position relative to the upstream TATA element in the promoter. Three TATA signal elements have been located previously and collectively are referred to here as the TATA box. A candidate p53 response element was identified between nucleotides 1,650 and 1,700 upstream from the TATA box. As indicated, one full p53 response element (three deviations from the consensus) along with two half sites (two mismatches from consensus), one on either side of the full element, have been identified. The mismatched nucleotides are indicated by the asterisk (*). (B) An electrophoretic mobility-shift assay demonstrates sequence-specific DNA binding of bacterially purified p53 to the identified _XPC_-p53 response element in vitro. Lane 1 indicates binding of p53 to a probe containing the p53 response element in the p21 gene. Lane 2 is free biotinylated probe containing the XPC p53 response element, and lane 3 is the XPC probe with recombinant p53. No band shift was observed and required the activating p53 antibody, pAB421, to observe a sequence-specific mobility shift (lane 4). Fiftyfold unlabeled oligo was used to compete out the band shift (lane 5), whereas a mutant oligo containing a 4-base interchange (see Experimental Procedures for sequence) was unable to achieve this competition (lane 6). Furthermore, addition of another p53 antibody (DO-1) was able to supershift the complex further (lane 7), adding strength to the specificity of the complex. Lane 8 shows absence of binding to the mutant oligo. Binding is noted also when the probe comprises both the full and two half p53-binding sites (XPCL, lane 9), whereas a similar length probe containing another potential p53-binding site in intron 3 (XPCintron3) is not bound by p53 (lane 10). In lanes where band shifts were observed, larger complexes were noted also, potentially because of further oligomerizations. The top-most band is the well, and the excess free probe at the bottom of the gel is not shown here.
Figure 6
Putative binding sites for UV-inducible transcription factors in the XPC gene. The sequence positions are indicated relative to the TATA box in the XPC promoter. The shaded boxes designate the potential binding sequences for the OCT-1, AP-1, and EGR-1 transcription factors. The EGR-1 putative binding sequence in the XPC gene contains one deviation from the consensus.
Similar articles
- p53 responsive nucleotide excision repair gene products p48 and XPC, but not p53, localize to sites of UV-irradiation-induced DNA damage, in vivo.
Fitch ME, Cross IV, Ford JM. Fitch ME, et al. Carcinogenesis. 2003 May;24(5):843-50. doi: 10.1093/carcin/bgg031. Carcinogenesis. 2003. PMID: 12771027 - Tumor suppressor p53 dependent recruitment of nucleotide excision repair factors XPC and TFIIH to DNA damage.
Wang QE, Zhu Q, Wani MA, Wani G, Chen J, Wani AA. Wang QE, et al. DNA Repair (Amst). 2003 May 13;2(5):483-99. doi: 10.1016/s1568-7864(03)00002-8. DNA Repair (Amst). 2003. PMID: 12713809 - Increased expression of p53 enhances transcription-coupled repair and global genomic repair of a UVC-damaged reporter gene in human cells.
Dregoesc D, Rybak AP, Rainbow AJ. Dregoesc D, et al. DNA Repair (Amst). 2007 May 1;6(5):588-601. doi: 10.1016/j.dnarep.2006.11.008. Epub 2006 Dec 28. DNA Repair (Amst). 2007. PMID: 17196445 - Xeroderma pigmentosum and molecular cloning of DNA repair genes.
Boulikas T. Boulikas T. Anticancer Res. 1996 Mar-Apr;16(2):693-708. Anticancer Res. 1996. PMID: 8687116 Review. - Cancer predisposition in mutant mice defective in multiple genetic pathways: uncovering important genetic interactions.
Meira LB, Reis AM, Cheo DL, Nahari D, Burns DK, Friedberg EC. Meira LB, et al. Mutat Res. 2001 Jun 2;477(1-2):51-8. doi: 10.1016/s0027-5107(01)00097-5. Mutat Res. 2001. PMID: 11376686 Review.
Cited by
- Mutant p53 reactivation restricts the protumorigenic consequences of wild type p53 loss of heterozygosity in Li-Fraumeni syndrome patient-derived fibroblasts.
Agarwal H, Tal P, Goldfinger N, Chattopadhyay E, Malkin D, Rotter V, Attery A. Agarwal H, et al. Cell Death Differ. 2024 Jul;31(7):855-867. doi: 10.1038/s41418-024-01307-4. Epub 2024 May 14. Cell Death Differ. 2024. PMID: 38745079 Free PMC article. - Aberrant ATM signaling and homology-directed DNA repair as a vulnerability of p53-mutant GBM to AZD1390-mediated radiosensitization.
Chen J, Laverty DJ, Talele S, Bale A, Carlson BL, Porath KA, Bakken KK, Burgenske DM, Decker PA, Vaubel RA, Eckel-Passow JE, Bhargava R, Lou Z, Hamerlik P, Harley B, Elmquist WF, Nagel ZD, Gupta SK, Sarkaria JN. Chen J, et al. Sci Transl Med. 2024 Feb 14;16(734):eadj5962. doi: 10.1126/scitranslmed.adj5962. Epub 2024 Feb 14. Sci Transl Med. 2024. PMID: 38354228 - Effect of ubiquitin protease system on DNA damage response in prostate cancer (Review).
Lin Y, Jin X. Lin Y, et al. Exp Ther Med. 2023 Nov 24;27(1):33. doi: 10.3892/etm.2023.12321. eCollection 2024 Jan. Exp Ther Med. 2023. PMID: 38125344 Free PMC article. Review. - The DNA damage response to radiological imaging: from ROS and γH2AX foci induction to gene expression responses in vivo.
López-Riego M, Płódowska M, Lis-Zajęcka M, Jeziorska K, Tetela S, Węgierek-Ciuk A, Sobota D, Braziewicz J, Lundholm L, Lisowska H, Wojcik A. López-Riego M, et al. Radiat Environ Biophys. 2023 Aug;62(3):371-393. doi: 10.1007/s00411-023-01033-4. Epub 2023 Jun 19. Radiat Environ Biophys. 2023. PMID: 37335333 Free PMC article. - Triptolide enhances carboplatin-induced apoptosis by inhibiting nucleotide excision repair (NER) activity in melanoma.
Wang G, Guo H, Ren Y, Chen W, Wang Y, Li J, Liu H, Xing J, Zhang Y, Li N. Wang G, et al. Front Pharmacol. 2023 Jun 1;14:1157433. doi: 10.3389/fphar.2023.1157433. eCollection 2023. Front Pharmacol. 2023. PMID: 37324464 Free PMC article.
References
- Ford J M, Hanawalt P C. J Biol Chem. 1997;272:28073–28080. - PubMed
- Ford J M, Baron E L, Hanawalt P C. Cancer Res. 1998;58:599–603. - PubMed
- Bowman K K, Sicard D M, Ford J M, Hanawalt P C. Mol Carcinog. 2000;29:17–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous