Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro - PubMed (original) (raw)
Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro
Alexandre Soulard et al. Mol Cell Biol. 2002 Nov.
Abstract
In Saccharomyces cerevisiae, the WASP (Wiskott-Aldrich syndrome protein) homologue Las17p (also called Bee1p) is an important component of cortical actin patches. Las17p is part of a high-molecular-weight protein complex that regulates Arp2/3 complex-dependent actin polymerization at the cell cortex and that includes the type I myosins Myo3p and Myo5p and verprolin (Vrp1p). To identify other factors implicated with this complex in actin regulation, we isolated proteins that bind to Las17p by two-hybrid screening and affinity chromatography. Here, we report the characterization of Lsb7/Bzz1p (for Las seventeen binding protein 7), an Src homology 3 (SH3) domain protein that interacts directly with Las17p via a polyproline-SH3 interaction. Bzz1p coimmunoprecipitates in a complex with Las17p, Vrp1p, Myo3/5p, Bbc1p, Hsp70p, and actin. It colocalizes with cortical actin patches and with Las17p. This localization is dependent on Las17p, but not on F-actin. Bzz1p interacts physically and genetically with type I myosins. While deletion of BZZ1 shows no obvious phenotype, simultaneous deletion of the BZZ1, MYO3, and MYO5 genes is lethal. Overexpression of Bzz1p inhibits cell growth, and a bzz1Delta myo5Delta double mutant is unable to restore actin polarity after NaCl stress. Finally, Bzz1p in vitro is able to recruit a functional actin polymerization machinery through its SH3 domains. Its interactions with Las17p, Vrp1p, and the type I myosins are essential for this process. This suggests that Bzz1p could be implicated in the regulation of actin polymerization.
Figures
FIG. 1.
Las17p interacts with Bzz1p in vivo and in vitro. (A) Two-hybrid interaction between Las17p and portions of Bzz1p. pACTII vectors carrying different GAL4-AD-BZZ1 gene fusions were transformed into Y190 cells expressing _GAL4_-DB in fusion with full-length LAS17. For each combination, extracts of three transformants were assayed for β-galactosidase activity. Interactions stronger than those of negative controls are shown as “+.” Empty vectors were used as negative controls, and the DB-ACT1 AD-PFY1 vector was used as a positive control. DB, DNA-binding domain; AD, activation domain. (B) Bzz1p interacts directly with Las17p in vitro. GST fused to full-length Bzz1p (GST-Bzz1p), GST fused to the COOH-terminal 188 aa of Bzz1p containing the two SH3 domains (GST-SH3), and six His fused to full-length Las17p (6His-Las17p) were purified from bacteria (see Materials and Methods). The same amount of GST fusion proteins (GST-Bzz1p or GST-SH3SH3) or GST alone bound to glutathione-Sepharose beads was incubated with a fixed amount of purified 6His-Las17p. After washing, total (T), bound (B), and unbound (UB) fractions were analyzed by Western blotting with Ni-NTA-HRP conjugate to reveal the 6His-Las17p fusion protein. (C) Two-hybrid interactions between Bzz1p and fragments of Las17p. pASΔΔ vectors containing different segments of LAS17 fused with GAL4-DB were transformed into Y190 cells expressing GAL4-AD in fusion with full-length BZZ1. Activity was assayed as in panel A.
FIG. 2.
Bzz1p associates with Las17p in cell extracts. High-speed extracts from RLY1 wild-type cells or RLY650 cells carrying Las17-protein A were prepared as previously described (30). Extracts were bound to IgG-Sepharose, washed, eluted with 0.5 M acetic acid (pH 3.6), and then electrophoresed on an 8% polyacrylamide gel. The result shown is a Coomassie blue-stained gel that contains molecular weight markers (M), control untagged wild-type extract (C), or Las17-protein A extract (B). The identities of the proteins determined by mass spectroscopy analysis of gel band eluates are indicated.
FIG. 3.
Bzz1p overexpression inhibits cell growth in a temperature-dependent manner. CLW001 wild-type strain containing either empty p424-GAL1 (pEmpty) or p424-GAL1-BZZ1 plasmid (p_BZZ1_) was grown overnight at 30°C in the selective liquid medium −Trp dextrose synthetic medium (SD). Ten-fold dilutions of about 106 cells containing empty proGAL vector plasmid or _proGAL-BZZ1_plasmid were spotted on SD-Trp or SG-Trp plates (dilutions from top to bottom for each temperature). Plates were incubated at 25 and 37°C for 2 days and then photographed.
FIG. 4.
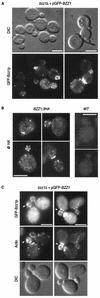
GFP-Bzz1p localizes in cortical patches partially overlapping with actin. (A) Localization of Bzz1p in living FY cells. pGFP-Nfus-BZZ1 vector encoding an NH2-terminal GFP-Bzz1p fusion protein was introduced into strain FSW701K to obtain strain FSW711K.Cells were grown to the exponential phase in dextrose synthetic medium (SD) −Ura liquid medium at 25°C and then harvested. After washing, they were visualized under a fluorescence microscope. DIC, differential interference contrast. (B) Immunolocalization of Bzz1-HAp. FSW71HAK (Bzz1-HAp) and CLW001 (wild type [_WT_]) strains were grown to the exponential phase in liquid YPD medium at 30°C. Cells were then fixed and subjected to indirect immunostaining with mouse monoclonal anti-HA primary antibody (@ HA) and Alexa-Fluor 568-labeled goat anti-mouse antibody (see Materials and Methods). Cells were visualized under a fluorescence microscope with a rhodamine filter. (C) Colocalization of GFP-Bzz1p and cortical actin patches. FSW711K cells were grown as in panel A, fixed, and stained with Alexa-phalloidin (see Materials and Methods). The arrows show examples of overlapping fluorescence between GFP and Alexa-phalloidin. Bar, 5 μm.
FIG. 5.
GFP-Bzz1p colocalization with Las17p and delocalization in a _las17_Δ mutant. (A) GFP-Bzz1p and Las17p colocalize in S. cerevisiae. A strain expressing GFP-Bzz1p (FSW711K) was grown to the exponential phase in dextrose synthetic medium (SD) −Ura liquid medium at 30°C. Cells were then fixed and incubated with polyclonal rabbit anti-Las17p primary antibody (@ Las17p), no antibody, or with Alexa-Fluor 568-labeled goat anti-rabbit secondary antibody. Cells were then visualized in a fluorescence microscope for GFP (GFP-Bzz1p) and Alexa 568 (@ Las17p, no @Las17p). The arrows show examples of overlapping fluorescence between GFP and Alexa 568. (B) FSW711K (_bzz1_Δ [left panel]) and FSW727KH (_bzz1_Δ _las17_Δ [right panel]) strains that contain pGFP-Nfus-BZZ1 were grown to the exponential phase in SD −Ura liquid medium at 25°C. An aliquot of harvested cells was washed in PBS buffer and observed with a fluorescence microscope. (C) Persistence of actin structures. An aliquot of the _bzz1_Δ _las17_Δ cells from panel A was washed in PBS, fixed, and stained with Alexa 568-phalloidin, and actin structures were visualized under a fluorescence microscope. (D) Anti-GFP Western blot analysis of whole-cell protein extracts from FSW711K (_bzz1_Δ) and FSW727KH (_bzz1_Δ _las17_Δ) strains. Strains were grown as in panel A, harvested, and washed in RIPA extraction buffer with protease inhibitors. Total protein extracts were prepared as described in Materials and Methods. Equal amounts of extract (20 μg) were subjected to SDS-PAGE. After transfer, the membrane was probed with rabbit anti-GFP antibody and revealed with HRP-conjugated goat anti-rabbit antibody. Bar, 5 μm.
FIG. 6.
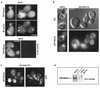
GFP-Bzz1p localized independently of actin. (A) Localization of Bzz1p after treatment of cells with latrunculin A (Lat A). Strain FSW711K was grown to the exponential phase at 30°C in liquid −Ura synthetic medium. An aliquot of these cells was then incubated at 30°C. in selective synthetic medium containing 200 μM (final concentration) latrunculin A in DMSO or in DMSO alone. Images in panel A show cells that were briefly fixed and stained with Alexa-phalloidin after 15 min of exposure to latrunculin A (or DMSO). (B) Localization of Bzz1p after latrunculin A removal. Cells from panel A were incubated for 45 min with latrunculin A (or DMSO), extensively washed, and allowed to regenerate for 120 min in fresh medium. Cells were then briefly fixed and stained for polymerized actin. All cells were visualized with the appropriate filters under a fluorescence microscope (see Materials and Methods). Bar, 5 μm.
FIG. 7.
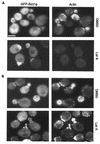
Salt stress sensitivity of a _bzz1_Δ _myo5_Δ double-deletion mutant. (A) SLW001 (wild type [WT]), SLW501T (_myo5_Δ), SLW701K (_lsb7_Δ), SLW571TK (_myo5_Δ _lsb7_Δ), and SLW572TK (_myo5_Δ _bzz1_Δ + pRS416-BZZ1) were grown in YPD to the exponential phase at 25°C. The same number of cells for each culture was then spotted on YPD, YPD-1 M NaCl, YPD-1.5 M KCl, YPD-0.5 M LiCl, and YPD-2 M sorbitol plates, which were incubated for 3 days at 25°C and then photographed. (B) Effect of NaCl on the actin cytoskeleton organization of a _myo5_Δ _bzz1_Δ double-disrupted strain. Strains used in panel A were grown in liquid YPD at 25°C. At 0 h, an equal volume of liquid YPD containing 2 M NaCl was added to each culture (final salt concentration, 1 M), and incubation continued at 25°C. The photos shown in panel B represent the four cell types fixed with formaldehyde and stained with Alexa-phalloidin at time points (t) 0, 1, and 6 h after addition of NaCl. Bar, 5 μm. (C) Quantification of actin cytoskeleton defects after salt stress. For each strain in panel B and at each time point (0, 1, and 6 h), more than 100 cells were counted. Black bars represent total budding cells, shaded bars represent budding cells with polarized actin patches, and white bars represent cells with aberrant actin structures. The results obtained were presented as a bar graph in which the horizontal axis represents different strains and the vertical axis represents the percentage of the total cell number.
FIG. 8.
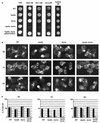
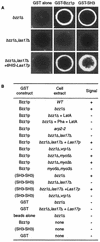
Bzz1p recruits the actin polymerization machinery in vitro. (A) Glutathione-Sepharose beads coated with either GST or GST fused to full-length Bzz1p or the two C-terminal SH3 domains of Bzz1p were incubated with extracts from either the _bzz1_Δ cells (FSW701K, upper panel) or the _bzz1_Δ _las17_Δ cells (FSW717KH middle panel) in the presence of Alexa-actin. Samples were incubated at room temperature, and the fluorescent signal was visualized. The photos shown below indicate representative positive (+) and negative (−) signals. In the lower panel (_bzz1_Δ _las17_Δ + 6His-Las17p), GST fusion protein-coated beads were first incubated with an excess of purified 6His-Las17p (see Materials and Methods) for 20 min. After extensive washing, the beads were tested for the actin polymerization assay by using the _lsb7_Δ _las17_Δ-derived extract. Panel B summarizes the results obtained and shows the results from experiments with extracts from wild-type (WT [SLW001]), _arp2_-2 (FKW201), _bzz1_Δ _vrp1_Δ (FSW751KK), _bzz1_Δ _myo5_Δ (SLW571KT), _bzz1_Δ _myo3_Δ (SLW371HK), and _myo5_Δ _myo3_Δ (RLY822) strains. Latrunculin A (LatA) was added to the _bzz1_Δ + LatA sample at the 0-h incubation. For the _bzz1_Δ + Pha + LatA sample, actin filament polymerized in the absence of beads was stabilized with phalloidin (Pha) prior to the addition of GST-Bzz1p-coated beads and latrunculin A.
FIG. 9.
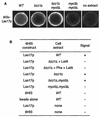
Las17p can also recruit the actin polymerization machinery in vitro_._ (A) Ni-NTA-agarose beads coated with six His fused to full-length Las17p were incubated in the presence of small amounts of Alexa-labeled actin with extracts from either the wild-type (WT) strain (CLW001), the _bzz1_Δ strain (FSW701K), the _bzz1_Δ _myo5_Δ strain (CLW571KT), or the _myo3_Δ _myo5_Δ strain (RLY822) or without yeast extract. After 10 to 20 min of incubation at room temperature, the fluorescence signal was visualized. The photos shown below indicate representative positive (+) and negative (−) signals. Panel B summarizes the results obtained.
Similar articles
- The WASP/Las17p-interacting protein Bzz1p functions with Myo5p in an early stage of endocytosis.
Soulard A, Friant S, Fitterer C, Orange C, Kaneva G, Mirey G, Winsor B. Soulard A, et al. Protoplasma. 2005 Oct;226(1-2):89-101. doi: 10.1007/s00709-005-0108-4. Epub 2005 Oct 20. Protoplasma. 2005. PMID: 16231105 - The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization.
Anderson BL, Boldogh I, Evangelista M, Boone C, Greene LA, Pon LA. Anderson BL, et al. J Cell Biol. 1998 Jun 15;141(6):1357-70. doi: 10.1083/jcb.141.6.1357. J Cell Biol. 1998. PMID: 9628892 Free PMC article. - Verprolin: a cool set of actin-binding sites and some very HOT prolines.
Munn AL, Thanabalu T. Munn AL, et al. IUBMB Life. 2009 Jul;61(7):707-12. doi: 10.1002/iub.195. IUBMB Life. 2009. PMID: 19507265 Review. - Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.
Higgs HN, Pollard TD. Higgs HN, et al. Annu Rev Biochem. 2001;70:649-76. doi: 10.1146/annurev.biochem.70.1.649. Annu Rev Biochem. 2001. PMID: 11395419 Review.
Cited by
- GreedyPlus: An Algorithm for the Alignment of Interface Interaction Networks.
Law B, Bader GD. Law B, et al. Sci Rep. 2015 Jul 13;5:12074. doi: 10.1038/srep12074. Sci Rep. 2015. PMID: 26165520 Free PMC article. - The WASP/Las17p-interacting protein Bzz1p functions with Myo5p in an early stage of endocytosis.
Soulard A, Friant S, Fitterer C, Orange C, Kaneva G, Mirey G, Winsor B. Soulard A, et al. Protoplasma. 2005 Oct;226(1-2):89-101. doi: 10.1007/s00709-005-0108-4. Epub 2005 Oct 20. Protoplasma. 2005. PMID: 16231105 - Finding friends and enemies in an enemies-only network: a graph diffusion kernel for predicting novel genetic interactions and co-complex membership from yeast genetic interactions.
Qi Y, Suhail Y, Lin YY, Boeke JD, Bader JS. Qi Y, et al. Genome Res. 2008 Dec;18(12):1991-2004. doi: 10.1101/gr.077693.108. Epub 2008 Oct 2. Genome Res. 2008. PMID: 18832443 Free PMC article. - The WASP and WAVE family proteins.
Kurisu S, Takenawa T. Kurisu S, et al. Genome Biol. 2009;10(6):226. doi: 10.1186/gb-2009-10-6-226. Epub 2009 Jun 15. Genome Biol. 2009. PMID: 19589182 Free PMC article. Review. - Phosphoinositide-binding interface proteins involved in shaping cell membranes.
Takenawa T. Takenawa T. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(5):509-23. doi: 10.2183/pjab.86.509. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20467216 Free PMC article. Review.
References
- Anton, I. M., W. Lu, B. J. Mayer, N. Ramesh, and R. S. Geha. 1998. The Wiskott-Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck. J. Biol. Chem. 273:20992-20995. - PubMed
- Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. - PubMed
- Ayscough, K. R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D. G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399-416. (Erratum, 146:1201, 1999.) - PMC - PubMed
- Barylko, B., D. D. Binns, and J. P. Albanesi. 2000. Regulation of the enzymatic and motor activities of myosin I. Biochim. Biophys. Acta 1496:23-35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous