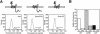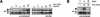Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened - PubMed (original) (raw)
Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened
James K Chen et al. Genes Dev. 2002.
Abstract
The steroidal alkaloid cyclopamine has both teratogenic and antitumor activities arising from its ability to specifically block cellular responses to vertebrate Hedgehog signaling. We show here, using photoaffinity and fluorescent derivatives, that this inhibitory effect is mediated by direct binding of cyclopamine to the heptahelical bundle of Smoothened (Smo). Cyclopamine also can reverse the retention of partially misfolded Smo in the endoplasmic reticulum, presumably through binding-mediated effects on protein conformation. These observations reveal the mechanism of cyclopamine's teratogenic and antitumor activities and further suggest a role for small molecules in the physiological regulation of Smo.
Figures
Figure 1
A photoaffinity derivative of cyclopamine cross-links Smo. (A) Chemical structure of PA-cyclopamine and its inhibitory activity on Shh signaling. (B) Upon photoactivation, 125I-labeled PA-cyclopamine cross-links two forms of Smo fused at the C terminus to Myc epitopes (Smo–Myc3) in COS-1 cells, and this labeling is inhibited by 1.5 μM KAAD-cyclopamine (left panel). Nontransfected cells and SmoA1–Myc3-expressing cells do not yield specifically cross-linked products. Western analysis with an anti-Myc antibody demonstrates that Smo–Myc3 and SmoA1–Myc3 expression levels are comparable and are not affected by KAAD-cyclopamine treatment (right panel). (C) The two Smo–Myc3 forms represent different glycosylation states, as one is endo H-sensitive (open arrowhead) and the other endo H-resistant (solid arrowhead). SmoA1–Myc3 is exclusively observed as an endo H-sensitive form. Phosphatase treatment did not alter the mobilities of Smo–Myc3 or SmoA1–Myc3 proteins (data not shown). (D) Endo H-sensitivity is indicative of ER localization, as confirmed by the colocalization of SmoA1–YFP (pseudocolored green, top left panel) and an ER marker (pseudocolored red, top middle panel; merge, top right panel) in C3H/10T1/2 cells. Cells expressing both SmoA1–YFP (pseudocolored green, bottom left panel) and a Golgi marker (pseudocolored red, bottom middle panel) exhibit no colocalization (merge, bottom right panel). (E) KAAD-cyclopamine abrogates Smo–Myc3/PA-cyclopamine cross-linking in a manner that is consistent with its inhibitory activity in the Shh-LIGHT2 assay (left panel) without altering cellular levels of Smo–Myc3 (right panel). Both ER and post-ER forms of Smo–Myc3 are depicted as described above. Cross-linking of an endogenous 160-kD protein (B) was competed by KAAD-cyclopamine only at concentrations significantly higher than those required for pathway inhibition (data not shown).
Figure 2
A fluorescent derivative of cyclopamine binds Smo-expressing cells. (A) Chemical structure of BODIPY-cyclopamine and its inhibitory activity on Shh signaling. (B) BODIPY-cyclopamine binds to a subpopulation of COS-1 cells transfected with a Smo expression construct, and KAAD-cyclopamine inhibits this interaction (KAAD-cyclopamine concentrations shown in boldface type). (C) Specific BODIPY-cyclopamine binding to Smo-expressing COS-1 cells can also be detected by flow cytometry (black trace; left and right panels), as this subpopulation exhibits high fluorescence intensity (brackets). In contrast, cells expressing SmoA1 (blue trace; left panel), mouse Ptch (green trace; left panel), mouse Frizzled 7 (red trace; right panel), or Drosophila Smo (blue trace; right panel) fail to bind BODIPY-cyclopamine in a specific manner. (D) Flow cytometric quantitation of specific BODIPY-cyclopamine binding to Smo-expressing cells (bracket; left panel) can be used to determine the affinities of Smo ligands through binding competitions (black trace, 0 nM; orange trace, 80 nM; red trace, 3 μM KAAD-cyclopamine; left panel), yielding an apparent _K_D of 23 nM for the KAAD-cyclopamine/Smo complex (right panel).
Figure 3
Cyclopamine binds to the heptahelical bundle in Smo. (A) COS-1 cells expressing either SmoΔCRD (middle panel) or SmoΔCT (right panel) were treated with BODIPY-cyclopamine and analyzed by flow cytometry. As with Smo-expressing cells (left panel), a subpopulation of these cells exhibit specific BODIPY-cyclopamine binding (see brackets). (B) BODIPY-binding to these cells is inhibited by 150 nM KAAD-cyclopamine to similar extents.
Figure 4
KAAD-cyclopamine binds to SmoA1 and promotes its exit from the ER. (A) Smo–GFP is localized to the plasma membrane and cytoplasmic vesicles of C3H/10T1/2 cells. The ER localization of SmoA1–GFP in C3H/10T1/2 cells is reversed by 10 μM KAAD-cyclopamine or 1 μM SAG, a Hh pathway agonist that directly binds Smo. (B) Glycosylation states of SmoA1–Myc3 upon treatment with 5 μM KAAD-cyclopamine include both endo H-sensitive (open arrowhead) and endo H-resistant (solid arrowhead) forms.
Figure 5
Ptch activity promotes cyclopamine/Smo complexation. (A) PA-cyclopamine cross-linking of post-ER Smo–Myc3 (solid arrowhead) in COS-1 cells is significantly increased upon Ptch expression in a dose-dependent manner (left panel). The labeling of ER-localized Smo–Myc3 (open arrowhead; left panel) is not affected by Ptch expression, and overall Smo–Myc3 expression levels remain constant (right panel). (B) ShhN reverses the effects of Ptch expression on PA-cyclopamine/Smo cross-linking.
Similar articles
- Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine.
Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Taipale J, et al. Nature. 2000 Aug 31;406(6799):1005-9. doi: 10.1038/35023008. Nature. 2000. PMID: 10984056 - Response to inhibition of smoothened in diverse epithelial cancer cells that lack smoothened or patched 1 mutations.
Galimberti F, Busch AM, Chinyengetere F, Ma T, Sekula D, Memoli VA, Dragnev KH, Liu F, Johnson KC, Guo Y, Freemantle SJ, Andrew AS, Greninger P, Robbins DJ, Settleman J, Benes C, Dmitrovsky E. Galimberti F, et al. Int J Oncol. 2012 Nov;41(5):1751-61. doi: 10.3892/ijo.2012.1599. Epub 2012 Aug 22. Int J Oncol. 2012. PMID: 22923130 Free PMC article. - Roughing up Smoothened: chemical modulators of hedgehog signaling.
King RW. King RW. J Biol. 2002 Nov 6;1(2):8. doi: 10.1186/1475-4924-1-8. J Biol. 2002. PMID: 12437769 Free PMC article. Review. - Impact of the Smoothened inhibitor, IPI-926, on smoothened ciliary localization and Hedgehog pathway activity.
Peluso MO, Campbell VT, Harari JA, Tibbitts TT, Proctor JL, Whitebread N, Conley JM, White KF, Kutok JL, Read MA, McGovern K, Faia KL. Peluso MO, et al. PLoS One. 2014 Mar 7;9(3):e90534. doi: 10.1371/journal.pone.0090534. eCollection 2014. PLoS One. 2014. PMID: 24608250 Free PMC article. - I only have eye for ewe: the discovery of cyclopamine and development of Hedgehog pathway-targeting drugs.
Chen JK. Chen JK. Nat Prod Rep. 2016 May 4;33(5):595-601. doi: 10.1039/c5np00153f. Nat Prod Rep. 2016. PMID: 26787175 Free PMC article. Review.
Cited by
- Trim46 contributes to the midbrain development via Sonic Hedgehog signaling pathway in zebrafish embryos.
Jung J, Kim J, Huh TL, Rhee M. Jung J, et al. Anim Cells Syst (Seoul). 2021 Mar 1;25(1):56-64. doi: 10.1080/19768354.2021.1889661. Anim Cells Syst (Seoul). 2021. PMID: 33717417 Free PMC article. - Interferon gamma and sonic hedgehog signaling are required to dysregulate murine neural stem/precursor cells.
Walter J, Hartung HP, Dihné M. Walter J, et al. PLoS One. 2012;7(8):e43338. doi: 10.1371/journal.pone.0043338. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952668 Free PMC article. - Examining the developmental toxicity of piperonyl butoxide as a Sonic hedgehog pathway inhibitor.
Rivera-González KS, Beames TG, Lipinski RJ. Rivera-González KS, et al. Chemosphere. 2021 Feb;264(Pt 1):128414. doi: 10.1016/j.chemosphere.2020.128414. Epub 2020 Sep 23. Chemosphere. 2021. PMID: 33007564 Free PMC article. Review. - Cross-talk between Human Papillomavirus Oncoproteins and Hedgehog Signaling Synergistically Promotes Stemness in Cervical Cancer Cells.
Vishnoi K, Mahata S, Tyagi A, Pandey A, Verma G, Jadli M, Singh T, Singh SM, Bharti AC. Vishnoi K, et al. Sci Rep. 2016 Sep 28;6:34377. doi: 10.1038/srep34377. Sci Rep. 2016. PMID: 27678330 Free PMC article. - The Hedgehog signalling pathway regulates autophagy.
Jimenez-Sanchez M, Menzies FM, Chang YY, Simecek N, Neufeld TP, Rubinsztein DC. Jimenez-Sanchez M, et al. Nat Commun. 2012;3:1200. doi: 10.1038/ncomms2212. Nat Commun. 2012. PMID: 23149744 Free PMC article.
References
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of Hedgehog target genes in Drosophila is mediated directly by the Cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes & Dev. 1996;10:2003–2013. - PubMed
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by Hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. - PubMed
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins N, Chen JK, Cooper MK, Taipale J, Olson JM, et al. Medulloblastoma growth inhibition by Hedgehog pathway blockade. Science. 2002;297:1559–1561. - PubMed
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. - PubMed
- Binns W, James LF, Shupe JL, Thacker EJ. Cyclopian-type malformations in lambs. Arch Environ Health. 1962;5:106–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous