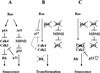Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence - PubMed (original) (raw)
Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence
Xianghong Zou et al. Genes Dev. 2002.
Abstract
A large number of human cancers display alterations in the Ink4a/cyclin D/Cdk4 genetic pathway, suggesting that activation of Cdk4 plays an important role in oncogenesis. Here we report that Cdk4-null mouse embryonic fibroblasts are resistant to transformation in response to Ras activation with dominant-negative (DN) p53 expression or in the Ink4a/Arf-null background, judged by foci formation, anchorage-independent growth, and tumorigenesis in athymic mice. Cdk4-null fibroblasts proliferate at normal rates during early passages. Whereas Cdk4(+/+)Ink4a/Arf(-/-) cells are immortal in culture, Cdk4(-/-)Ink4a/Arf(-/-) cells undergo senescence during continuous culture, as do wild-type cells. Activated Ras also induces premature senescence in Cdk4(-/-)Ink4a/Arf(-/-) cells and Cdk4(-/-) cells with DNp53 expression. Thus, Cdk4 deficiency causes senescence in a unique Arf/p53-independent manner, which accounts for the loss of transformation potential. Cdk4-null cells express high levels of p21(Cip1/Waf1) with increased protein stability. Suppression of p21(Cip1/Waf1) by small interfering RNA (siRNA), as well as expression of HPV-E7 oncoprotein, restores immortalization and Ras-mediated transformation in Cdk4(-/-)Ink4a/Arf(-/-) cells and Cdk4(-/-) cells with DNp53 expression. Therefore, Cdk4 is essential for immortalization, and suppression of Cdk4 could be a prospective strategy to recruit cells with inactive Arf/p53 pathway to senescence.
Figures
Figure 1
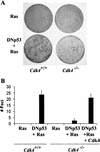
Cdk4-null mouse embryonic fibroblasts are resistant to transformation induced by expression of H-Rasval12 and dominant-negative p53. (A) Passage 4 mouse embryonic fibroblasts (MEFs) with the indicated genotypes were infected with a retrovirus encoding H-Rasval12, or with a virus encoding H-Rasval12 and a dominant-negative p53 (DNp53; amino acids 275–368) with the internal ribosomal entry site. To restore Cdk4 in _Cdk4_−/− MEFs, cells were infected with a Cdk4 retrovirus 48 h prior to H-Rasval12 + DNp53. Cells were then cultured in the medium containing 5% FBS for 21 d. (B) The numbers of foci per 60-mm dish in the assays are expressed as means + S.E.M. from three independent MEF preparations.
Figure 2
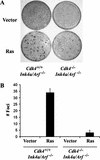
Cdk4−/−Ink4a/Arf−/− MEFs are resistant to HRasval12-induced transformation. (A) Passage 4 MEFs were infected with a retrovirus encoding H-Rasval12, or with a control virus with the pBabe-hygro vector. Cells were then cultured in the medium containing 5% FBS for 17 d. (B) The numbers of foci per 60-mm dish in the assays are expressed as means + S.E.M. from three independent MEF preparations.
Figure 3
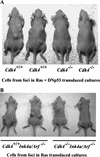
_Cdk4_-null embryonic fibroblasts isolated from foci are not tumorigenic in athymic mice. (A) Foci were isolated from the confluent cultures at 21 d following retrovirus transduction of H-Rasval12 and DNp53 (see Fig. 1). (B) Foci were isolated from the confluent cultures at 17 d following H-Rasval-12 expression (see Fig. 2). Cells were then expanded, and injected into athymic mice (106 cells per site). Mice were examined 21 d after injection.
Figure 4
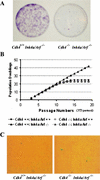
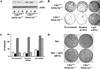
Cdk4 disruption renders cells insensitive to immortalization associated with Ink4a/Arf deficiency. (A) MEFs at passage 11 were plated at a low density (1 × 103 cells per 60-mm dish), and cultured for 10 d. Colonies grown from isolated cells were stained with crystal violet. (B) Primary MEFs with indicated genotypes were propagated in culture according to the 3T3 protocol. Accumulated numbers of population doublings are shown. The data represent experiments using three independent MEF preparations for each genotype. (C) Senescence-associated β-galactosidase (SAβ-gal) staining. MEFs at passage 12 were inoculated at 3 × 103 cells per 60-mm dish, and 10 d later, the cells were stained for SAβ-gal, as described in Materials and Methods.
Figure 5
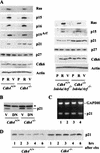
_Cdk4_-null MEFs express high levels of p21Cip1/Waf1 with increased stability regardless of the Arf/p53 status. (A) Cells with indicated genotypes were infected with retrovirus constructed from pBabe-H-Rasval12 or pBabe-hygro control vector. Infected cells were selected for 72 h in the presence of 50 μg/mL hygromycin, and were then analyzed by immunoblotting for the proteins indicated. P, uninfected proliferating cells (no selection); R, cells infected with H-Rasval12 retrovirus; V, cells infected with vector control virus. (B) Cells were infected with retrovirus constructed from LXSN-dominant-negative (DN) p53 or LXSN control vector (V). Infected cells were selected for 72 h in the presence of 2 μg/mL puromycin, and then analyzed by immunoblotting for the expression of p21Cip1/Waf1. *, a band with nonspecific immunoreactivity. (C) Expression of p21Cip1/Waf1 mRNA is unaltered. Exponentially proliferating cells at passage 4 were analyzed by RT–PCR for the expression of p21Cip1/Waf1 mRNA and GAPDH mRNA. The genotypes of cells: 1, Cdk4+/+ (wild-type); 2, _Cdk4_−/−; 3, Cdk4+/+_Ink4a/Arf_−/−; 4, _Cdk4_−/−_Ink4a/Arf_−/−. (D) p21Cip1/Waf1 is stabilized in _Cdk4_−/− cells. Exponentially proliferating cells were treated with 40 μg/mL cycloheximide (chx) for the times indicated, and cellular levels of p21Cip1/Waf1 were determined by immunoblotting. These data represent experiments using three independent cell preparations at passage 3 or 4 for each genotype.
Figure 6
Suppression of p21Cip1/Waf1 expression by siRNA restores immortalization and transformation in _Cdk4_−/−_Ink4a/Arf_−/− MEFs. (A) Cells at passage 10 were transfected with siRNA that specifically targets p21Cip1/Waf1 mRNA or with random double-stranded (ds) RNA. Cellular expression of p21Cip1/Waf1 was analyzed by immunoblotting at 72 h after transfection. p, nontransfected proliferating cells; c, cells transfected with control random dsRNA; si, anti-p21Cip1/Waf1 siRNA. (B) Cells at passage 10 were transfected with the anti-p21 siRNA or control dsRNA, and 24 h later plated at a density of 1 × 103 cells/plate. (C) Colonies (>2 mm) were counted at 10 d postplating, and the numbers are expressed as means + S.E.M. from three independent cell preparations. Open columns, Cdk4+/+; closed columns, _Cdk4_−/−; hatched columns, Cdk4+/+_Ink4a/Arf_−/−; dotted columns, _Cdk4_−/−_Ink4a/Arf_−/−. (D) Cells at passage 4 were transfected with the anti-p21Cip1/Waf1 siRNA or control dsRNA, and 24 h later infected with H-Rasval-12 retrovirus. Foci formation was scored at 15 d posttransfection.
Figure 7
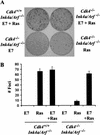
Human papillomavirus E7 oncoprotein restores Ras-mediated transformation of _Cdk4_−/−_Ink4a/Arf_−/− MEFs. (A) Passage 4 MEFs with indicated genotypes were infected with E7 retrovirus or control virus, followed by infection with H-Rasval-12 retrovirus or control virus at a 24-h interval. Cells were then cultured in the medium containing 5% FBS for 17 d. (B) The numbers of foci per 60-mm dish in the assays are expressed as means + S.E.M. from three independent MEF preparations.
Figure 8
Effects of Cdk4 disruption on the pathways controlling senescence and transformation. (A) Senescence response of wild-type cells to Ras activation. (B) Ras-induced transformation with inactivated Ink4a/Arf/p53 pathway. (C) Senescence rendered by Cdk4 disruption.
Similar articles
- Sequential extension of proliferative lifespan in human fibroblasts induced by over-expression of CDK4 or 6 and loss of p53 function.
Morris M, Hepburn P, Wynford-Thomas D. Morris M, et al. Oncogene. 2002 Jun 20;21(27):4277-88. doi: 10.1038/sj.onc.1205492. Oncogene. 2002. PMID: 12082615 - Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.
Shin J, Yang J, Lee JC, Baek KH. Shin J, et al. Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963 - Defects in TGF-beta signaling overcome senescence of mouse keratinocytes expressing v-Ha-ras.
Tremain R, Marko M, Kinnimulki V, Ueno H, Bottinger E, Glick A. Tremain R, et al. Oncogene. 2000 Mar 23;19(13):1698-709. doi: 10.1038/sj.onc.1203471. Oncogene. 2000. PMID: 10763827 - Control of p53 ubiquitination and nuclear export by MDM2 and ARF.
Zhang Y, Xiong Y. Zhang Y, et al. Cell Growth Differ. 2001 Apr;12(4):175-86. Cell Growth Differ. 2001. PMID: 11331246 Review. - [Cyclin dependent kinase inhibitors and replicative senescence].
Gerland LM, Ffrench M, Magaud JP. Gerland LM, et al. Pathol Biol (Paris). 2001 Dec;49(10):830-9. doi: 10.1016/s0369-8114(01)00249-8. Pathol Biol (Paris). 2001. PMID: 11776695 Review. French.
Cited by
- D-type Cyclins are important downstream effectors of cytokine signaling that regulate the proliferation of normal and neoplastic mammary epithelial cells.
Zhang Q, Sakamoto K, Wagner KU. Zhang Q, et al. Mol Cell Endocrinol. 2014 Jan 25;382(1):583-592. doi: 10.1016/j.mce.2013.03.016. Epub 2013 Apr 4. Mol Cell Endocrinol. 2014. PMID: 23562856 Free PMC article. Review. - Combination strategies to overcome drug resistance in FLT+ acute myeloid leukaemia.
Yang J, Friedman R. Yang J, et al. Cancer Cell Int. 2023 Aug 11;23(1):161. doi: 10.1186/s12935-023-03000-x. Cancer Cell Int. 2023. PMID: 37568211 Free PMC article. - Palbociclib Effectively Halts Proliferation but Fails to Induce Senescence in Patient-Derived Glioma Stem Cells.
Morris-Hanon O, Marazita MC, Romorini L, Isaja L, Fernandez-Espinosa DD, Sevlever GE, Scassa ME, Videla-Richardson GA. Morris-Hanon O, et al. Mol Neurobiol. 2019 Nov;56(11):7810-7821. doi: 10.1007/s12035-019-1633-z. Epub 2019 May 23. Mol Neurobiol. 2019. PMID: 31124078 - Murine muscle cell models for Pompe disease and their use in studying therapeutic approaches.
Takikita S, Myerowitz R, Zaal K, Raben N, Plotz PH. Takikita S, et al. Mol Genet Metab. 2009 Apr;96(4):208-17. doi: 10.1016/j.ymgme.2008.12.012. Epub 2009 Jan 22. Mol Genet Metab. 2009. PMID: 19167256 Free PMC article. - Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR Pathways Induces a Synergistic Anti-Tumor Effect in Malignant Pleural Mesothelioma Cells.
Bonelli MA, Digiacomo G, Fumarola C, Alfieri R, Quaini F, Falco A, Madeddu D, La Monica S, Cretella D, Ravelli A, Ulivi P, Tebaldi M, Calistri D, Delmonte A, Ampollini L, Carbognani P, Tiseo M, Cavazzoni A, Petronini PG. Bonelli MA, et al. Neoplasia. 2017 Aug;19(8):637-648. doi: 10.1016/j.neo.2017.05.003. Epub 2017 Jul 11. Neoplasia. 2017. PMID: 28704762 Free PMC article.
References
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. - PubMed
- Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
- Boylan JF, Sharp DM, Leffet L, Bowers A, Pan W. Analysis of site-specific phosphorylation of the retinoblastoma protein during cell cycle progression. Exp Cell Res. 1999;248:110–114. - PubMed
- Carnero A, Hudson JD, Price CM, Beach DH. p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat Cell Biol. 2000;2:148–155. - PubMed
- Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18:4808–4818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous