Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways - PubMed (original) (raw)
Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways
Wolfgang W Leitner et al. Nat Med. 2003 Jan.
Abstract
Cancer vaccines targeting 'self' antigens that are expressed at consistently high levels by tumor cells are potentially useful in immunotherapy, but immunological tolerance may block their function. Here, we describe a novel, naked DNA vaccine encoding an alphavirus replicon (self-replicating mRNA) and the self/tumor antigen tyrosinase-related protein-1. Unlike conventional DNA vaccines, this vaccine can break tolerance and provide immunity to melanoma. The vaccine mediates production of double-stranded RNA, as evidenced by the autophosphorylation of dsRNA-dependent protein kinase R (PKR). Double-stranded RNA is critical to vaccine function because both the immunogenicity and the anti-tumor activity of the vaccine are blocked in mice deficient for the RNase L enzyme, a key component of the 2',5'-linked oligoadenylate synthetase antiviral pathway involved in double-stranded RNA recognition. This study shows for the first time that alphaviral replicon-encoding DNA vaccines activate innate immune pathways known to drive antiviral immune responses, and points the way to strategies for improving the efficacy of immunization with naked DNA.
Figures
Fig. 1
Design of plasmids used for this study. a, The pSin1.5 vector containing the genes for the mouse or human TRP-1 antigen. b, Two conventional plasmids (pcDNA3 and pSport), encoding mouse and human TRP-1, respectively. In case of the pSin vector, self-replicating RNA (replicon) is transcribed under control of a CMV promoter. This replicon encodes the replicase gene and the tumor-associated antigen. After translation, more RNA copies are produced in the transfected cell through the action of the replicase–enzyme complex, (non-structural proteins, NSP). c, Antigen expression was confirmed in vitro by transfection of BHK-21 cells and western blot using sera from mice immunized with vaccinia-mTRP-1. Lane 1, B16.F10 melanoma; lane 2, CMV-mTRP-1; lane 3, pCMV-hTRP-1; lane 4, pSin-mTRP-1; lane 5, pSin-hTRP-1; lane 6, pCMV-EGFP. d, pCMV-mTRP-1 is a functional vaccine in Tyrp1_−/−, but is unable to immunize (that is, break tolerance in) wild-type (Figs. 2 and 3_a) or Tyrp1 _+/_− mice. Serum (diluted 1:5, 1:25, 1:125, 1:625) was analyzed by ELISA after 5 immunizations. Shown is the average of 5 mice after subtraction of background values from naïve mice. P value at 1:125 by Wilcoxon rank sum test is 0.051.
Fig. 2
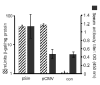
Antigen expression does not correlate with immunogenicity. After delivery by gene gun, we observed no difference in the expression of a reporter gene (β-gal) encoded on either a conventional (pCMV) or replicase-based (pSin) plasmid (hatched bars). Skin biopsies of 3 mice each were harvested 24 h after immunization with a single shot (theoretical dose = 1 μg plasmid). Results are shown as mUnits β-gal with s.e.m. (left scale). Similarly, no significant differences in antigen levels were detected after intradermal injection of the plasmids (10 μg each; data not shown). Average serum antibody titers (n = 4) after gene gun immunization with DNA plasmids encoding mouse TRP-1 (right scale) or a control plasmid (co_n_ = pSin-β-gal) are shown as filled bars with standard error. Mice were bled 1 week after the last immunization and antibody titers were measured by ELISA. Sera were diluted 1:125 and measured individually. The experiment was repeated 4 times with sera from independently immunized mice yielding comparable results. Results shown are from a representative experiment.
Fig. 3
Tumor prevention by immunization with various plasmids encoding TRP-1. a, Mice were immunized 5 times at weekly intervals by gene gun and challenged subcutaneously with 1 × 105 B16.F10 melanoma cells. Shown is the compiled percentage of tumor-free mice 21 d after challenge. Tumors were measured for 3 weeks after challenge in a blinded fashion. Total number of mice per group was between 10 (β-gal plasmids and pCMV-mTRP-1) and 50 (all others). Mice immunized with pSin-mTRP-1 were also protected after challenge with a tumor inoculum of 5 × 105 B16 cells (data not shown). b, Vitiligo is only induced by immunization with a conventional vector encoding human TRP-1, but not with a replicase-based vector encoding either human or mouse TRP-1. Shown is one representative mouse for each vaccine, 10 days after 5 weekly immunizations. Depigmentation begins at the site of immunization with the gene gun (shaved abdomen). c, Impact of the depletion of CD4+ or CD8+ cells at the effector phase (pre-challenge). Results are calculated from the average tumor size 21 d after challenge and based on the average size of tumors in non-depleted mice. Significant increases in tumor size after depletion with monoclonal antibody are indicated by arrows (Wilcoxon rank sum test). The reduction in the number of tumor-free mice after antibody depletion is shown as percentage of tumor-free mice based on the number of tumor-free mice in the non-depleted
Fig. 4
Production of biologically active dsRNA in cells transfected with replicase-based plasmids. PKR is activated by dsRNA in the lysate of transfected cells and autophosphorylates. pSin-EGFP or pCMV-EGFP transfected, sorted SW480 cells were lysed and a kinase assay was performed with purified GST, GST fused to wild-type PKR (GST–PKR), or GST fused to the dominant-negative K296 mutant of PKR (PKR-con) in the presence of [γ-32P]ATP. The ‘No PKR’ lane contains no added proteins and is a measure of endogenous kinase levels. Proteins were separated by SDS–PAGE and activated; autophosphorylated PKR was detected by autoradiography. Non-transfected cells (con) were obtained from transfection cultures to account for any transfection-induced PKR activation. The results obtained with SW480 cells were confirmed in BHK-21 cells using a different preparation of recombinant PKR protein (non-GST, data not shown).
Fig. 5
The involvement of dsRNA and dsRNA-dependent pathways in the immune response to replicase-based plasmids. a, Rnasel_−/_− mice and Rnasel _+/_− littermates were immunized weekly for 5 weeks (♦) and were bled before challenge together with non-immunized controls (⋄). Sera were diluted 1:5, 1:25, 1:125 and 1:625, and measured individually. Shown is the average antibody titer (n = 6) with standard errors. P value was calculated by ANOVA. The titer of antibody against TRP-1 was determined by ELISA as in Fig 2. b, To confirm the validity of the animal model, Rnasel _+/_− and Rnasel_−/_− mice were immunized twice with vaccinia-mTRP-1 (•). Two weeks after the second immunization, mice were bled together with non-immunized controls (○). The experiment was repeated with identical results.
Fig. 6
The involvement of dsRNA and dsRNA-dependent pathways in the immune response after immunization with replicase-based plasmids. a, Tumors are effectively prevented by pSin-mTRP-1 in Rnasel _+/_− (•), but not in Rnasel_−/_− mice (○). Shown are the average tumor sizes of mice (n = 6) immunized weekly with pSin-mTRP-1 5 times and challenged with B16.F10 7–10 days after the last immunization. No difference was observed in the tumor growth between Rnasel_−/_− and Rnasel _+/_− control mice immunized with pSin-β-gal (data not shown). The experiment was repeated with the same result. b, Average tumor sizes of Rnasel_−/_− (□) and Rnasel _+/_− (▪) mice immunized twice with recombinant vaccinia-mTRP-1 and challenged with B16 melanoma subcutaneously 2 weeks after the second immunization. Shown is the average tumor size of at least 6 mice. *Naive control mice. c, Compiled percentage of tumor-free mice (n = 12) immunized with replicase-based plasmid 21 d after subcutaneous challenge with B16.F10 melanoma. Mice were challenged 7–10 days after the last immunization. Shown are pooled data from two independent experiments. d, Tumor protection of Rnasel_−/_−mice receiving adoptively transferred CD8+ T cells from donors immunized with pSin-mTRP-1 (□). Challenge-control Rnasel_−/_− mice were only lymphocyte-depleted, but received no treatment (▪). Treatment-control mice were lymphocyte-depleted and treated in the same way as the experimental group with IL-2 and pSin-mTRP-1, but received purified CD8+ T cells from naive donors (▪). The difference between the experimental group and both control groups (n = 10 for all experiments) was statistically significant (P = 0.012 for challenge control, P = 0.027 for treatment control, by ANOVA). There was no difference between the two control groups (P = 0.526). In addition, there was a statistically significant difference (P < 0.05) between the group receiving immune CD8+ T cells and the challenge control on every single day of measurement (as determined by the F test). The experiment was repeated in wild-type mice with comparable results (data not shown) establishing the functionality of immune CD8+ T cells in Rnasel_−/_− mice.
Similar articles
- Type I Interferons are essential for the efficacy of replicase-based DNA vaccines.
Leitner WW, Bergmann-Leitner ES, Hwang LN, Restifo NP. Leitner WW, et al. Vaccine. 2006 Jun 12;24(24):5110-8. doi: 10.1016/j.vaccine.2006.04.059. Epub 2006 May 6. Vaccine. 2006. PMID: 16725231 Free PMC article. - Comparison of two cancer vaccines targeting tyrosinase: plasmid DNA and recombinant alphavirus replicon particles.
Goldberg SM, Bartido SM, Gardner JP, Guevara-Patiño JA, Montgomery SC, Perales MA, Maughan MF, Dempsey J, Donovan GP, Olson WC, Houghton AN, Wolchok JD. Goldberg SM, et al. Clin Cancer Res. 2005 Nov 15;11(22):8114-21. doi: 10.1158/1078-0432.CCR-05-1410. Clin Cancer Res. 2005. PMID: 16299244 - Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity.
Avogadri F, Merghoub T, Maughan MF, Hirschhorn-Cymerman D, Morris J, Ritter E, Olmsted R, Houghton AN, Wolchok JD. Avogadri F, et al. PLoS One. 2010 Sep 10;5(9):e12670. doi: 10.1371/journal.pone.0012670. PLoS One. 2010. PMID: 20844763 Free PMC article. - Self-replicating alphavirus RNA vaccines.
Ljungberg K, Liljeström P. Ljungberg K, et al. Expert Rev Vaccines. 2015 Feb;14(2):177-94. doi: 10.1586/14760584.2015.965690. Epub 2014 Oct 1. Expert Rev Vaccines. 2015. PMID: 25269775 Review. - Alphavirus-based vaccines.
Lundstrom K. Lundstrom K. Curr Opin Mol Ther. 2002 Feb;4(1):28-34. Curr Opin Mol Ther. 2002. PMID: 11883692 Review.
Cited by
- Immunotherapeutic advances in glioma management: The rise of vaccine-based approaches.
Andrew Awuah W, Shah MH, Tan JK, Ranganathan S, Sanker V, Darko K, Tenkorang PO, Adageba BB, Ahluwalia A, Shet V, Aderinto N, Kundu M, Abdul-Rahman T, Atallah O. Andrew Awuah W, et al. CNS Neurosci Ther. 2024 Sep;30(9):e70013. doi: 10.1111/cns.70013. CNS Neurosci Ther. 2024. PMID: 39215399 Free PMC article. Review. - Initiation of a ZAKα-dependent ribotoxic stress response by the innate immunity endoribonuclease RNase L.
Xi J, Snieckute G, Martínez JF, Arendrup FSW, Asthana A, Gaughan C, Lund AH, Bekker-Jensen S, Silverman RH. Xi J, et al. Cell Rep. 2024 Apr 23;43(4):113998. doi: 10.1016/j.celrep.2024.113998. Epub 2024 Mar 28. Cell Rep. 2024. PMID: 38551960 Free PMC article. - Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment.
Pampeno C, Opp S, Hurtado A, Meruelo D. Pampeno C, et al. Int J Mol Sci. 2024 Mar 2;25(5):2925. doi: 10.3390/ijms25052925. Int J Mol Sci. 2024. PMID: 38474178 Free PMC article. Review. - Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy.
Pampeno C, Hurtado A, Opp S, Meruelo D. Pampeno C, et al. Int J Mol Sci. 2023 Oct 6;24(19):14948. doi: 10.3390/ijms241914948. Int J Mol Sci. 2023. PMID: 37834397 Free PMC article. Review.
References
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. - PubMed
- Blum JS, Ma C, Kovats S. Antigen-presenting cells and the selection of immunodominant epitopes. Crit Rev Immunol. 1997;17:411–417. - PubMed
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. - PubMed
- Leitner WW, Hammerl P, Thalhamer J. Nucleic acid for the treatment of cancer: Genetic vaccines and DNA adjuvants. Curr Pharm Res. 2001;7:1641–1667. - PubMed
- Sasaki S, Amara RR, Oran AE, Smith JM, Robinson HL. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nature Biotechnol. 2001;19:543–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 BC010763-01/Intramural NIH HHS/United States
- CA44059/CA/NCI NIH HHS/United States
- Z99 CA999999/Intramural NIH HHS/United States
- R01-AI34039/AI/NIAID NIH HHS/United States
- R01 AI034039/AI/NIAID NIH HHS/United States
- R01 CA044059/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials