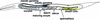An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans - PubMed (original) (raw)
An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans
Michael A Miller et al. Genes Dev. 2003.
Abstract
During sexual reproduction in most animals, oocytes arrest in meiotic prophase and resume meiosis (meiotic maturation) in response to sperm or somatic cell signals. Despite progress in delineating mitogen-activated protein kinase (MAPK) and CDK/cyclin activation pathways involved in meiotic maturation, it is less clear how these pathways are regulated at the cell surface. The Caenorhabditis elegans major sperm protein (MSP) signals oocytes, which are arrested in meiotic prophase, to resume meiosis and ovulate. We used DNA microarray data and an in situ binding assay to identify the VAB-1 Eph receptor protein-tyrosine kinase as an MSP receptor. We show that VAB-1 and a somatic gonadal sheath cell-dependent pathway, defined by the CEH-18 POU-class homeoprotein, negatively regulate meiotic maturation and MAPK activation. MSP antagonizes these inhibitory signaling circuits, in part by binding VAB-1 on oocytes and sheath cells. Our results define a sperm-sensing control mechanism that inhibits oocyte maturation, MAPK activation, and ovulation when sperm are unavailable for fertilization. MSP-domain proteins are found in diverse animal taxa, where they may regulate contact-dependent Eph receptor signaling pathways.
Figures
Figure 1
Oocyte meiotic maturation in the adult hermaphrodite gonad. The anterior (left) and posterior (right) gonad arms are drawn to depict the germ line and somatic gonadal sheath cells, respectively. Sperm are stored in the spermatheca and use MSP to signal oocyte meiotic maturation and sheath cell contraction, which act together to facilitate ovulation. Meiotic maturation and ovulation occur in an assembly line-like fashion in the proximal gonad. Nuclear envelope breakdown of the maturing oocyte is indicated by dotted lines. The oocyte is fertilized during ovulation as it enters the spermatheca, and embryos develop in the uterus until they are laid through the vulva (not labeled). In hermaphrodites, spermatogenesis takes place in the gonad arms and sperm enter the spermatheca during the first ovulation. Spermatogenesis does not occur in fog-2(q71) females, so mating is required for reproduction. Sperm are inseminated through the vulva during mating and then crawl to the spermatheca.
Figure 2
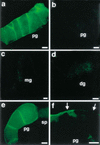
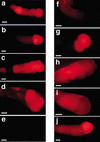
In situ binding of MSP-FITC. (a) Two-hundred nanomolar MSP-FITC binds to oocyte and sheath cell membranes of the proximal gonad arms of mated fog-2(q71) females. (b) Binding to membranes in the proximal gonad following preincubation with a 25-fold molar excess of unlabeled MSP is not observed. (c,d) Binding is not observed in the male gonad (c) or the distal hermaphrodite gonad (d). (e,f) MSP-FITC binds to oocytes and sheath cell membranes in control emo-1(oz1)/+ heterozygotes (e), but binding to oocyte membranes is significantly reduced or eliminated in emo-1(oz1) mutants (f), which have impaired germline secretory function. Binding to sheath cells, which have normal secretory function, is not eliminated (f, arrows). pg, proximal gonad; mg, male gonad; dg, distal hermaphrodite gonad; sp, spermatheca. Bars, 15 μ.
Figure 3
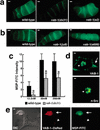
MSP binds to the VAB-1 Eph receptor. (a) Compared to wild-type gonads, MSP binding (200 nM MSP-FITC) is reduced in vab-1(dx31) null mutant gonads, but not in vab-1(e2) kinase-impaired gonads. Proximal is to the right. (b) MSP binding is not reduced in the ephrin signaling mutants vab-1(ju8) and vab-1(e699). (c) The binding intensity of vab-1(dx31) gonads is significantly lower than wild-type (*; P < 0.001). Binding intensity was measured at three MSP-FITC concentrations using arbitrary units that correspond to fluorescent intensity. vab-1(dx31) null mutants exhibit a reduction in saturable MSP binding. Because of the thinness of the sheath cells (∼0.2–1 μm), we were unable to separately quantify the reduction in sheath cell binding. Error bars indicate S.D. (d,e) Expressing VAB-1 in COS-7 cells is sufficient to confer MSP binding activity. (d) Two-hundred nanomolar MSP binds to the surface of live COS-7 cells (arrow) expressing VAB-1 (upper panel), but not n-Src (lower panel). (e) COS-7 cells expressing VAB-1∷DsRed bind MSP (arrows), whereas those that do not express VAB-1∷DsRed do not bind. Arrows in d and e indicate specific binding, and arrowheads indicate internal autofluorescence caused by the transfection procedure. Bars, 10 μ.
Figure 4
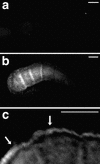
VAB-1∷GFP is expressed in oocytes and sheath cells. (a) Fluorescence is not observed in dissected, wild-type gonads. (b,c) VAB-1∷GFP expression is detectable in oocytes (b) and sheath cells (c) in gonads dissected from the transgenic strain juIs24. Images were processed using deconvolution software (see Materials and Methods). Bars, 25 μ.
Figure 5
VAB-1 and CEH-18 negatively regulate MAPK activation. The MAPK-YT antibody recognizes the diphosphorylated, active form of MAPK (red). Staining is not observed in mpk-1(ga117) MAPK null mutant gonads or unmated fog-2(q71) female gonads. (a) MAPK activation is not dependent on sperm in unc-24(e138) fem-3(e1996);emo-1(oz1) oocytes, which have impaired secretory function. (b) In wild-type hermaphrodites, MAPK activation is dependent on sperm, but activation occurs in the most proximal one to three oocytes. (c) In contrast, several oocytes contain activated MAPK in vab-1(dx31) hermaphrodites. (d,e) MAPK activation occurs in the absence of sperm in unmated fog-2(q71);ceh-18(mg57);vab-1 (RNAi) females (d), but not in unmated fog-2(q71);vab-1(dx31) females (e). (f,g) Unmated fog-2(q71);ceh-18(mg57) female gonads contain low levels of activated MAPK, but rarely in the most proximal oocytes (f). However, activation is observed following mating (g). (h,i) MAPK-YT staining in fog-2(q71);ceh-18(mg57);_vab-1(dx31)/_+ heterozygous females (h) is not affected by mating (i) to wild-type males. (j) efn-2(ev658) hermaphrodites exhibit slightly enhanced levels of MAPK-YT staining compared to wild-type hermaphrodites (b). Proximal is to the right. Bars, 20 μ.
Figure 6
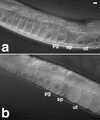
VAB-1 and CEH-18 inhibit oocyte meiotic maturation and ovulation. DIC micrographs of unmated fog-2(q71) (a) and fog-2(q71);ceh-18(mg57); vab-1(RNAi) (b) females. (a) The oocyte maturation and ovulation rate is very low in unmated fog-2(q71) females (see Table 2 for rate measurements). Oocytes are arrested in meiotic prophase and accumulate in the proximal gonad arm (pg) until insemination occurs and sperm migrate to the spermatheca (sp). Unfertilized oocytes do not accumulate in the uterus (ut). (b) In contrast, the oocyte maturation and ovulation rates are high and independent of sperm in unmated fog-2(q71); ceh-18(mg57); vab-1(RNAi) females. Oocytes do not accumulate in the proximal gonad arm in these mutants. Instead, the uterus fills with unfertilized oocytes. Bar, 10 μ.
Figure 7
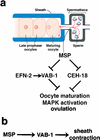
A sperm-sensing control mechanism regulates oocyte meiotic maturation and MAPK activation. (a) Sperm release MSP, which binds to VAB-1 and another receptor(s) on oocytes and sheath cells. MSP promotes oocyte M-phase entry (maturation), MAPK activation, and ovulation by antagonizing ephrin/Eph receptor (EFN-2/VAB-1) and sheath cell-dependent (CEH-18) inhibitory circuits (see Discussion for details; drawing modified with permission from Villeneuve 2001). (b) In sheath cells, MSP stimulates the basal contraction rate by acting as a VAB-1 agonist.
Similar articles
- Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans.
Govindan JA, Cheng H, Harris JE, Greenstein D. Govindan JA, et al. Curr Biol. 2006 Jul 11;16(13):1257-68. doi: 10.1016/j.cub.2006.05.020. Curr Biol. 2006. PMID: 16824915 - Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans.
Cheng H, Govindan JA, Greenstein D. Cheng H, et al. Curr Biol. 2008 May 20;18(10):705-714. doi: 10.1016/j.cub.2008.04.043. Epub 2008 May 8. Curr Biol. 2008. PMID: 18472420 Free PMC article. - Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation.
Corrigan C, Subramanian R, Miller MA. Corrigan C, et al. Development. 2005 Dec;132(23):5225-37. doi: 10.1242/dev.02083. Epub 2005 Nov 2. Development. 2005. PMID: 16267094 - The multifaceted C. elegans major sperm protein: an ephrin signaling antagonist in oocyte maturation.
Kuwabara PE. Kuwabara PE. Genes Dev. 2003 Jan 15;17(2):155-61. doi: 10.1101/gad.1061103. Genes Dev. 2003. PMID: 12533505 Review. No abstract available. - Developmental control of oocyte maturation and egg activation in metazoan models.
Von Stetina JR, Orr-Weaver TL. Von Stetina JR, et al. Cold Spring Harb Perspect Biol. 2011 Oct 1;3(10):a005553. doi: 10.1101/cshperspect.a005553. Cold Spring Harb Perspect Biol. 2011. PMID: 21709181 Free PMC article. Review.
Cited by
- The genetics and cell biology of fertilization.
Geldziler BD, Marcello MR, Shakes DC, Singson A. Geldziler BD, et al. Methods Cell Biol. 2011;106:343-75. doi: 10.1016/B978-0-12-544172-8.00013-X. Methods Cell Biol. 2011. PMID: 22118284 Free PMC article. Review. - Canonical RTK-Ras-ERK signaling and related alternative pathways.
Sundaram MV. Sundaram MV. WormBook. 2013 Jul 11:1-38. doi: 10.1895/wormbook.1.80.2. WormBook. 2013. PMID: 23908058 Free PMC article. Review. - Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development.
Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Lee MH, et al. Genetics. 2007 Dec;177(4):2039-62. doi: 10.1534/genetics.107.081356. Genetics. 2007. PMID: 18073423 Free PMC article. - Reevaluation of the role of LIP-1 as an ERK/MPK-1 dual specificity phosphatase in the C. elegans germline.
Das D, Seemann J, Greenstein D, Schedl T, Arur S. Das D, et al. Proc Natl Acad Sci U S A. 2022 Jan 18;119(3):e2113649119. doi: 10.1073/pnas.2113649119. Proc Natl Acad Sci U S A. 2022. PMID: 35022236 Free PMC article. - Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor.
Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Noren NK, et al. Biochem J. 2009 Aug 27;422(3):433-42. doi: 10.1042/BJ20090014. Biochem J. 2009. PMID: 19552627 Free PMC article.
References
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. - PubMed
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. - PubMed
- Bottino D, Mogilner A, Roberts T, Stewart M, Oster G. How nematode sperm crawl. J Cell Sci. 2002;115:367–384. - PubMed
- Boxem M, Srinivasan DG, van den Heuvel S. The Caenorhabditis elegans gene ncc-1 encodes a CDC2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development. 1999;126:2227–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM57173/GM/NIGMS NIH HHS/United States
- R01 GM049882/GM/NIGMS NIH HHS/United States
- R01 GM065115/GM/NIGMS NIH HHS/United States
- P30CA68485/CA/NCI NIH HHS/United States
- GM49882/GM/NIGMS NIH HHS/United States
- R01 GM057173/GM/NIGMS NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 CA009592/CA/NCI NIH HHS/United States
- T32 CA09592/CA/NCI NIH HHS/United States
- R56 GM057173/GM/NIGMS NIH HHS/United States
- GM65115/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous