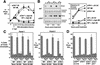Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance - PubMed (original) (raw)
Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance
Eric J Brown et al. Genes Dev. 2003.
Abstract
A Cre/lox-conditional mouse line was generated to evaluate the role of ATR in checkpoint responses to ionizing radiation (IR) and stalled DNA replication. We demonstrate that after IR treatment, ATR and ATM each contribute to early delay in M-phase entry but that ATR regulates a majority of the late phase (2-9 h post-IR). Double deletion of ATR and ATM eliminates nearly all IR-induced delay, indicating that ATR and ATM cooperate in the IR-induced G2/M-phase checkpoint. In contrast to the IR-induced checkpoint, checkpoint delay in response to stalled DNA replication is intact in ATR knockout cells and ATR/ATM and ATR/p53 double-knockout cells. The DNA replication checkpoint remains intact in ATR knockout cells even though the checkpoint-stimulated inhibitory phosphorylation of Cdc2 on T14/Y15 and activating phosphorylation of the Chk1 kinase no longer occur. Thus, incomplete DNA replication in mammalian cells can prevent M-phase entry independently of ATR and inhibitory phosphorylation of Cdc2. When DNA replication inhibitors are removed, ATR knockout cells proceed to mitosis but do so with chromosome breaks, indicating that ATR provides a key genome maintenance function in S phase.
Figures
Figure 1
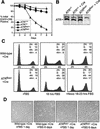
Generation of a floxed conditional allele of ATR (_ATR_flox). (A) Schematic representations of the targeting vector, wild-type ATR locus, recombined locus, and the final conditional allele of ATR (_ATR_flox) are shown. Following confirmation by Southern blot, the ES cells with a recombined ATR allele were transfected with the pCAGGS-FLPe expression vector (Buchholz et al. 1998) to remove the selection cassette. Removal of the selection cassette results in the _lox_-conditional (_ATR_flox) allele shown. (B) Confirmation of the _ATR_flox allele by Southern blot. DNA prepared from ES cell clones was digested with _Bam_HI + _Kpn_I or _Hin_dIII alone and Southern-blotted for hybridization with the 5′ and 3′ probes indicated in A, respectively. DNA fragments representing wild-type, internally recombined, recombined, and _ATR_flox alleles are discussed in the text and indicated in A.
Figure 2
_ATR_Δ/− cells divide and then exit the cell cycle. (A) Asychronously expanding _ATR_flox/− MEFs expressing EGFP–Cre are selectively lost from culture. The cells that continue to express EGFP–Cre over the course of 8 d of growth were quantitated as a percentage of their initial representation 1 d after infection with lenti-EGFP–Cre. ▪, wild type; ♦, ATR+/−; ●, _ATR_flox/+; ▴, _ATR_flox/−. (B) Western blot quantitation of ATR protein levels in _ATR_flox/− and ATR+/− MEFs 60 h after NLS-Cre expression (lenti-Cre). (C) Cell cycle analysis of lenti-Cre-infected wild-type and _ATR_flox/− MEFs. DNA content was determined by propidium iodide staining and flow cytometric analysis. (D) Exit of _ATR_Δ/− cells from the cell cycle. Cells treated as in C are shown after 1 and 6 d of FBS stimulation. Cells were split 1:4 at day 3.
Figure 3
ATR and ATM cooperate in checkpoint responses to γ-irradiation. (A) Serum-deprived and lenti-Cre-infected wild-type, _ATR_flox/−, and _ATM_−/− MEFs were γ-irradiated (20 Gy) after 17 h of FBS stimulation and then treated with 0.5 μM nocodazole for 5 h (corresponding to 18–23 h of FBS stimulation). The percentage of cells exhibiting chromosome condensation (panel 1) and phosphorylated histone H3 (panel 2) were quantitated by chromosome spread preparation and immunostaining, respectively. Differences between the total percent of cells with condensed chromatin (panel 1) versus phosphohistone H3 staining (panel 2) is consistent with the phosphorylation of histone H3 occurring prior to condensation and remaining phosphorylated throughout M phase. (B) ATR is required for late-phase mitotic delay in response to IR. Entry into mitosis (without nocodazole treatment) was quantitated by phosphohistone H3 staining before and after treatment with 20 Gy of γ-radiation at 17 h of FBS stimulation. ▪, wild-type; ♦, _ATR_Δ/−; ●, wild-type + IR; ▴, _ATR_Δ/− + IR. (C) ATR and ATM cooperate in the cell cycle delay elicited by IR. Mitotic entry was assessed after IR exposure as in B to compare mitotic entry of _ATR_flox/− (no lenti-Cre infection), _ATM_−/−, and lenti-Cre-infected _ATR_flox/− and _ATR_flox/− _ATM_−/− (_ATR_Δ/− and _ATR_Δ/− _ATM_−/−) MEFs. _ATR_flox/− and _ATR_flox/− _ATM_−/− MEFs were derived from embryonic littermates. (D) ATR and ATM cooperate in p53 S18 phosphorylation. Lysates generated from lenti-Cre-infected wild-type, _ATR_flox/−, and _ATM_−/− cells at various times after IR treatment were Western-blotted and detected with a phospho-specific antibody to phospho-S18 p53. (E) Cells treated as described in C were analyzed for p53 S18 phosphorylation by Western blot detection as described in D. Error bars represent the standard deviation (S.D.) from the mean of values obtained in these experiments. nt, not treated with IR.
Figure 4
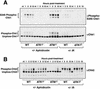
ATR loss does not eliminate the DNA replication checkpoint. (A) Both wild-type and _ATR_Δ/− cells are prevented from entering mitosis by aphidicolin (Aph) treatment. Cre-expressing wild-type and _ATR_flox/− cells were treated with aphidicolin upon S-phase entry following FBS stimulation (15 h post-FBS), and the percentage of cells entering mitosis was quantitated by phosphohistone H3 staining. ▪, wild-type; ♦, _ATR_Δ/−; ●, wild-type + Aph; ▴, _ATR_Δ/− + Aph. (B) Cyclin B1-associated H1 kinase activity in wild-type and _ATR_Δ/− cells. After 15 h of FBS stimulation, Cre-expressing wild-type and _ATR_flox/− cells were treated with aphidicolin (Aph), exposed to 20 Gy of γ-radiation (IR), or were left untreated (nt). Cells were then harvested 3, 6, and 9 h later, and immunoprecipitated cyclin B1-associated H1 kinase activity was assayed. Autoradiographs and PhosphorImager-quantitation (graph) of 32P-labeled H1 following SDS-PAGE are shown. Immunoprecipitates were also Western-blotted and detected for cyclin B1 to control for equivalent cyclin B1 recovery (Western: cyclin B1). ▪, wild-type; ♦, _ATR_Δ/−; ✖, wild-type + IR; ✚, _ATR_Δ/− + IR; ●, wild-type + Aph; ▴, _ATR_Δ/− + Aph. (C) Chromosome condensation is prevented by DNA replication inhibitors in lenti-Cre-infected wild-type and _ATR_flox/− cells. Aphidicolin (5 μM) or hydroxyurea (0.5 mM) was added 16 h after FBS stimulation. Following a 2-h incubation to allow cells already in G2 and M phase at the time of treatment to pass through to G1, cells were treated with nocodazole (0.5 μM) for 5 h (18–23 and 23–28 h of FBS stimulation). Mitotic cells were quantitated by chromosome condensation (panel 1) and histone H3 phosphorylation (panel 2). Note that quantitation by chromosome condensation includes mitotic cells with normal or fragmented (PCC) chromosome structure. (D) The DNA replication checkpoint is intact in _ATR_Δ/− _ATM_−/− and _ATR_Δ/− _p53_−/− cells. Mitotic entry was analyzed by phosphohistone H3 staining cells as described in C with nocodazole treatment for 5 h, from 18 to 23 h of FBS stimulation. Error bars represent the standard deviation (S.D.) from the mean of values obtained in these experiments.
Figure 5
Regulation of Chk1 and Chk2 by ATR and ATM, respectively. (A) Chk1 phosphorylation in response to either aphidicolin or IR requires ATR. After 15 h of FBS stimulation, Cre-expressing wild-type and _ATR_flox/− MEFs were treated with aphidicolin or IR. Lysates were prepared at various times posttreatment, and Western blots were detected for phospho-S345 Chk1 (upper panel) or total Chk1 (lower panel). (B) Chk2 phosphorylation in response to aphidicolin and IR is not dependent on ATR. Samples generated as in A were Western-blotted and detected for Chk2. The slower-migrating phosphorylated and faster-migrating unphosphorylated forms are indicated.
Figure 6
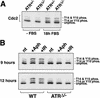
Cdc2 phosphorylation on T14 and Y15. (A) Cdc2 is phosphorylated normally as cells enter S phase. After 18 h of FBS stimulation, lysates from lenti-Cre-infected _ATR_flox/− cells were generated and subjected to SDS-PAGE and Western blot detection of Cdc2. The unphosphorylated (fastest-migrating), singly phosphorylated (phospho-T14 or phospho-Y15), and doubly phosphorylated (slowest-migrating, phospho-T14 and phospho-Y15) forms of Cdc2 are indicated. (B) Accumulation of phospho-T14/Y15 Cdc2 following aphidicolin or IR does not occur in _ATR_Δ/− cells. After 15 h of FBS stimulation, Cre-expressing wild-type and _ATR_flox/− cells were either left untreated (nt) or treated with either aphidicolin (Aph) or 20 Gy of γ-radiation (IR). Cells were then harvested 9 and 12 h after treatment, and lysates were Western-blotted and detected for Cdc2 as in A.
Figure 7
Aphidicolin causes DSBs in ATR-depleted cells, but DSBs do not prevent mitotic entry upon aphidicolin release. (A) Aphidicolin causes phosphorylation of H2AX in _ATR_Δ/− cells. At 15 h of FBS stimulation, lenti-Cre-infected wild-type and _ATR_flox/− cells were left untreated or treated with aphidicolin. Cells were harvested 1, 2, and 4 h later. Lysates were Western-blotted and detected for phosphohistone H2AX. (B) Schematic representation of an assay to quantitate the effect of DSBs on preventing mitotic entry and detect increased chromosome breakage upon aphidicolin treatment. (C) Mitotic entry of aphidicolin-treated wild-type and _ATR_Δ/− cells after aphidicolin release. After a 2-h aphidicolin treatment, Cre-expressing wild-type and _ATR_flox/− MEFs were washed four times with 10% FBS DMEM to remove aphidicolin. Two and a half hours later, cells were collected in mitosis by nocodazole treatment (0.5 μM) for 1.5 h. Chromosome spreads were prepared to quantitate the percentage of cells entering mitosis and to assess chromosome breakage as described in D and E below. Cells were released from or maintained in aphidicolin as indicated. (D) Mitotic spreads with differing degrees of chromosome breakage were observed and quantitated using the assay described in B and C. Examples of spreads with 1–10 breaks and >10 breaks are shown. (E) Aphidicolin induces breaks specifically in _ATR_Δ/− cells. Cre-expressing wild-type and _ATR_flox/− MEFs released from aphidicolin treatment as described in B and C (+Aph & Release) were analyzed for the frequency of each category of mitotic chromosome spread shown in D. The chromosome breakage observed in cells left untreated with aphidicolin is also shown (−Aph). N, normal chromosome spread. (F) Aphidicolin treatment does not cause apoptosis in _ATR_Δ/− cells. Cre-expressing wild-type and _ATR_flox/− MEFs left untreated, treated with aphidicolin, or treated with and released from aphidicolin were stained for Annexin V and quantitated by flow cytometry.
Similar articles
- ATM regulates ATR chromatin loading in response to DNA double-strand breaks.
Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O. Cuadrado M, et al. J Exp Med. 2006 Feb 20;203(2):297-303. doi: 10.1084/jem.20051923. Epub 2006 Feb 6. J Exp Med. 2006. PMID: 16461339 Free PMC article. - Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation.
Gatei M, Sloper K, Sorensen C, Syljuäsen R, Falck J, Hobson K, Savage K, Lukas J, Zhou BB, Bartek J, Khanna KK. Gatei M, et al. J Biol Chem. 2003 Apr 25;278(17):14806-11. doi: 10.1074/jbc.M210862200. Epub 2003 Feb 14. J Biol Chem. 2003. PMID: 12588868 - Involvement of the ATR- and ATM-dependent checkpoint responses in cell cycle arrest evoked by pierisin-1.
Shiotani B, Kobayashi M, Watanabe M, Yamamoto K, Sugimura T, Wakabayashi K. Shiotani B, et al. Mol Cancer Res. 2006 Feb;4(2):125-33. doi: 10.1158/1541-7786.MCR-05-0104. Mol Cancer Res. 2006. PMID: 16513843 - The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.
Smith J, Tho LM, Xu N, Gillespie DA. Smith J, et al. Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review. - DNA damage checkpoint, damage repair, and genome stability.
Liu WF, Yu SS, Chen GJ, Li YZ. Liu WF, et al. Yi Chuan Xue Bao. 2006 May;33(5):381-90. doi: 10.1016/S0379-4172(06)60064-4. Yi Chuan Xue Bao. 2006. PMID: 16722332 Review.
Cited by
- Two sides of the Myc-induced DNA damage response: from tumor suppression to tumor maintenance.
Campaner S, Amati B. Campaner S, et al. Cell Div. 2012 Feb 28;7(1):6. doi: 10.1186/1747-1028-7-6. Cell Div. 2012. PMID: 22373487 Free PMC article. - ATR activity controls stem cell quiescence via the cyclin F-SCF complex.
Salvi JS, Kang J, Kim S, Colville AJ, de Morrée A, Billeskov TB, Larsen MC, Kanugovi A, van Velthoven CTJ, Cimprich KA, Rando TA. Salvi JS, et al. Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2115638119. doi: 10.1073/pnas.2115638119. Epub 2022 Apr 27. Proc Natl Acad Sci U S A. 2022. PMID: 35476521 Free PMC article. - A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products.
Li K, Zhao S, Long J, Su J, Wu L, Tao J, Zhou J, Zhang J, Chen X, Peng C. Li K, et al. Cancer Cell Int. 2020 Jan 30;20:36. doi: 10.1186/s12935-020-1114-5. eCollection 2020. Cancer Cell Int. 2020. PMID: 32021565 Free PMC article. - Identification of ATR-Chk1 pathway inhibitors that selectively target p53-deficient cells without directly suppressing ATR catalytic activity.
Kawasumi M, Bradner JE, Tolliday N, Thibodeau R, Sloan H, Brummond KM, Nghiem P. Kawasumi M, et al. Cancer Res. 2014 Dec 15;74(24):7534-45. doi: 10.1158/0008-5472.CAN-14-2650. Epub 2014 Oct 21. Cancer Res. 2014. PMID: 25336189 Free PMC article. - ATR Kinase Inhibition Protects Non-cycling Cells from the Lethal Effects of DNA Damage and Transcription Stress.
Kemp MG, Sancar A. Kemp MG, et al. J Biol Chem. 2016 Apr 22;291(17):9330-42. doi: 10.1074/jbc.M116.719740. Epub 2016 Mar 3. J Biol Chem. 2016. PMID: 26940878 Free PMC article.
References
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. - PubMed
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. - PubMed
- Buchholz F, Angrand PO, Stewart AF. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat Biotechnol. 1998;16:657–662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous