Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin - PubMed (original) (raw)
Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin
Michela Rigoni et al. Br J Pharmacol. 2003 Mar.
Abstract
(1) Stimulation of the vanilloid receptor-1 (TRPV1) results in the activation of nociceptive and neurogenic inflammatory responses. Poor specificity and potency of TRPV1 antagonists has, however, limited the clarification of the physiological role of TRPV1. (2) Recently, iodo-resiniferatoxin (I-RTX) has been reported to bind as a high affinity antagonist at the native and heterologously expressed rat TRPV1. Here we have studied the ability of I-RTX to block a series of TRPV1 mediated nociceptive and neurogenic inflammatory responses in different species (including transfected human TRPV1). (3) We have demonstrated that I-RTX inhibited capsaicin-induced mobilization of intracellular Ca(2+) in rat trigeminal neurons (IC(50) 0.87 nM) and in HEK293 cells transfected with the human TRPV1 (IC(50) 0.071 nM). (4) Furthermore, I-RTX significantly inhibited both capsaicin-induced CGRP release from slices of rat dorsal spinal cord (IC(50) 0.27 nM) and contraction of isolated guinea-pig and rat urinary bladder (pK(B) of 10.68 and 9.63, respectively), whilst I-RTX failed to alter the response to high KCl or SP. (5) Finally, in vivo I-RTX significantly inhibited acetic acid-induced writhing in mice (ED(50) 0.42 micro mol kg(-1)) and plasma extravasation in mouse urinary bladder (ED(50) 0.41 micro mol kg(-1)). (6) In in vitro and in vivo TRPV1 activated responses I-RTX was approximately 3 log units and approximately 20 times more potent than capsazepine, respectively. This high affinity antagonist, I-RTX, may be an important tool for future studies in pain and neurogenic inflammatory models.
Figures
Figure 1
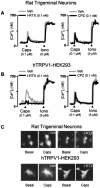
Typical tracings and microscopic images showing the inhibitory effect of iodo-resiniferatoxin (I-RTX, 0.1 n
M
) or capsazepine (CPZ, 0.1 n
M
) on capsaicin (0.1 μ
M
) induced Ca2+ mobilization in rat trigeminal neurons (A, C) and HEK293 cells transfected with the human TRPV1 (hTRPV1 HEK293; B, C).
Figure 2
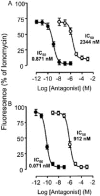
The inhibitory effect of iodo-resiniferatoxin (I-RTX, filled circles), capsazepine (CPZ, open circles) on capsaicin (0.1 μ
M
)-induced increases in cytoplasmic Ca2+ ion concentration in rat trigeminal neurons (A) and HEK293 cells transfected with the human TRPV1 (hTRPV1 HEK293; B). Each entry is the mean±s.e.mean of at least 80 cells; *P<0.05.
Figure 3
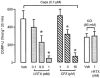
The inhibitory effect of iodo-resiniferatoxin (I-RTX, closed bars), capsazepine (CPZ, hatched bars) or the combination of their respective vehicles (empty bar) on the release of calcitonin gene-related peptide-like immunoreactivity (CGRP-LI) induced by capsaicin (0.1 μ
M
) or KCl (80 m
M
) from slices of rat dorsal spinal cord. Each entry is the mean±s.e.mean of at least five experiments; *P<0.05.
Figure 4
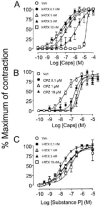
The effect of iodo-resiniferatoxin (I-RTX), capsazepine (CPZ) or their respective vehicles (empty circles) on the contraction produced by increasing concentrations of capsaicin (A, B) or substance P (C) (both given in a cumulative manner) in isolated guinea-pig bronchial rings. Each entry is the mean±s.e.mean of at least six experiments.
Figure 5
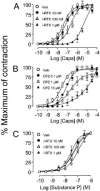
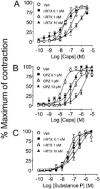
The effect of iodo-resiniferatoxin (I-RTX), capsazepine (CPZ) or their respective vehicles (empty circles) on the contraction produced by increasing concentrations of capsaicin (A, B) or substance P (C) (given in a cumulative manner) in isolated strips of guinea-pig urinary bladder. Each entry is the mean±s.e.mean of at least six experiments.
Figure 6
The effect of iodo-resiniferatoxin (I-RTX), capsazepine (CPZ) or their respective vehicles (empty circles) on the contraction produced by increasing concentrations of capsaicin (A, B) or substance P (C) (given in a cumulative manner) in isolated strips of rat urinary bladder. Each entry is the mean±s.e.mean of at least six experiments.
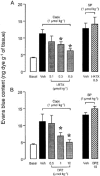
Figure 7
The effect of iodo-resiniferatoxin (I-RTX), capsazepine (CPZ) or the combination of their respective vehicles (black bars) on the Evans blue extravasation induced by the intravenous injection of capsaicin (Caps) or substance P (SP) in the mouse urinary bladder. Each entry is the mean±s.e.mean of at least six experiments; *P<0.05.
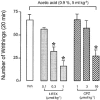
Figure 8
The inhibitory effect of iodo-resiniferatoxin (I-RTX), capsazepine (CPZ) or the combination of their respective vehicles (empty bar) on the number of writhing responses produced by intraperitoneal administration of acetic acid in mice. Each entry is the mean±s.e.mean of at least seven experiments; *P<0.05.
Similar articles
- Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin.
Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB. Seabrook GR, et al. J Pharmacol Exp Ther. 2002 Dec;303(3):1052-60. doi: 10.1124/jpet.102.040394. J Pharmacol Exp Ther. 2002. PMID: 12438527 - Halogenation of a capsaicin analogue leads to novel vanilloid TRPV1 receptor antagonists.
Appendino G, Harrison S, De Petrocellis L, Daddario N, Bianchi F, Schiano Moriello A, Trevisani M, Benvenuti F, Geppetti P, Di Marzo V. Appendino G, et al. Br J Pharmacol. 2003 Aug;139(8):1417-24. doi: 10.1038/sj.bjp.0705387. Br J Pharmacol. 2003. PMID: 12922928 Free PMC article. - Cloning and pharmacological characterization of mouse TRPV1.
Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. Correll CC, et al. Neurosci Lett. 2004 Nov 3;370(1):55-60. doi: 10.1016/j.neulet.2004.07.058. Neurosci Lett. 2004. PMID: 15489017 - The vanilloid (capsaicin) receptor: receptor types and species differences.
Szallasi A. Szallasi A. Gen Pharmacol. 1994 Mar;25(2):223-43. doi: 10.1016/0306-3623(94)90049-3. Gen Pharmacol. 1994. PMID: 8026721 Review. - Complex Regulation of TRPV1 by Vanilloids.
Szallasi A, Blumberg PM. Szallasi A, et al. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 6. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 6. PMID: 21204495 Free Books & Documents. Review.
Cited by
- Emerging Families of Ion Channels Involved in Urinary Bladder Nociception.
Araki I, Yoshiyama M, Kobayashi H, Mochizuki T, Du S, Okada Y, Takeda M. Araki I, et al. Pharmaceuticals (Basel). 2010 Jul 19;3(7):2248-2267. doi: 10.3390/ph3072248. Pharmaceuticals (Basel). 2010. PMID: 27713353 Free PMC article. Review. - Antitussive activity of iodo-resiniferatoxin in guinea pigs.
Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Trevisani M, et al. Thorax. 2004 Sep;59(9):769-72. doi: 10.1136/thx.2003.012930. Thorax. 2004. PMID: 15333853 Free PMC article. - A Review of the Effects of Pain and Analgesia on Immune System Function and Inflammation: Relevance for Preclinical Studies.
DeMarco GJ, Nunamaker EA. DeMarco GJ, et al. Comp Med. 2019 Dec 1;69(6):520-534. doi: 10.30802/AALAS-CM-19-000041. Epub 2019 Dec 20. Comp Med. 2019. PMID: 31896389 Free PMC article. Review. - Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure.
Sappington RM, Calkins DJ. Sappington RM, et al. Invest Ophthalmol Vis Sci. 2008 Jul;49(7):3004-17. doi: 10.1167/iovs.07-1355. Epub 2008 Mar 24. Invest Ophthalmol Vis Sci. 2008. PMID: 18362111 Free PMC article. - Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress.
Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, Calkins DJ. Sappington RM, et al. Channels (Austin). 2015;9(2):102-13. doi: 10.1080/19336950.2015.1009272. Channels (Austin). 2015. PMID: 25713995 Free PMC article.
References
- AMANN R., MAGGI C.A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. - PubMed
- BEVAN S., GEPPETTI P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. - PubMed
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous