Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes - PubMed (original) (raw)
Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes
Sylvia O Suadicani et al. Glia. 2003.
Abstract
Propagation of intercellular calcium waves (ICW) between astrocytes depends on the diffusion of signaling molecules through gap junction channels and diffusion through the extracellular space of neuroactive substances acting on plasmalemmal receptors. The relative contributions of these two pathways vary in different brain regions and under certain pathological conditions. We have previously shown that in wild-type spinal cord astrocytes, ICW are primarily gap junction-dependent, but that deletion of the main gap junction protein (Cx43) by homologous recombination results in a switch in mode of ICW propagation to a purinoceptor-dependent mechanism. Such a compensatory mechanism for ICW propagation was related to changes in the pharmacological profile of P2Y receptors, from an adenine-sensitive P2Y(1), in wild-type, to a uridine-sensitive P2U receptor subtype, in Cx43 knockout (KO) astrocytes. Using oligonucleotide antisense to Cx43 mRNA for acute downregulation of connexin43 expression levels, we provide evidence for the molecular nature of such compensatory mechanism. Pharmacological studies and Western blot analysis indicate that there is a reciprocal regulation of P2Y(1) and P2Y(4) expression levels, such that downregulation of Cx43 leads to decreased expression of the adenine-sensitive P2Y(1) receptor and increased expression of the uridine-sensitive P2Y(4) receptor. This change in functional expression of the P2Y receptor subtype population in acutely downregulated Cx43 was paralleled by changes in the mode of ICW propagation, similar to that previously observed for Cx43 KO spinal cord astrocytes. On the basis of these results, we propose that Cx43 regulates both modes of ICW by altering P2Y receptor subtype expression in addition to providing intercellular coupling.
Copyright 2003 Wiley-Liss, Inc.
Figures
Fig. 1
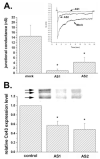
Decreased dye coupling between cortical astrocytes induced by treatment with Cx43 antisense oligonucleotides. A: Mean values of the relative changes in LY spread between astrocytes exposed for 48 h to increased concentrations of oligonucleotide antisense to the mRNA corresponding to the exon1 of Cx43 DNA; note that only at 3.0 μM did the antisense induce a significant reduction (about 40%) in dye coupling and that increasing antisense concentration to 6.0 μM did not further decrease LY spread. Changes in LY spread were normalized against the distance of LY spread obtained under control (untreated) conditions. B: Mean values of the relative changes in LY spread induced by 48-h exposure of cortical astrocytes to 3.0 μM oligonucleotides, one antisense to the mRNA corresponding to exon1 (AS1) and the other to exon2 (AS2) of Cx43 DNA; the two sense-oligonucleotides (S1 and S2) employed to control for nonspecific effects had the same but inverted sequence of nucleotides as the two antisense oligonucleotides (AS-ONs). Note that dye coupling between mock- and sense-treated astrocytes was not significantly different from that obtained for control, untreated cultures and that both AS-ONs significantly reduced LY spread to levels comparable to that observed in untreated cultures exposed to 100 μM carbenoxolone (carb; a gap junction channel blocker). An example of dye coupling observed in control and in AS1-treated cultures is shown on the micrograph below. The histograms bars correspond to the mean values and standard errors of 30 measurements of LY spread. *P < 0.001 (one-way analysis of variance [ANOVA], followed by the Tukey test).
Fig. 2
Changes in electrical coupling and in Cx43 expression levels induced by antisense oligonucleotides (AS-ONs). A: Histogram showing the mean values of junctional conductance measured from 10–12 pairs of cortical astrocytes derived from cultures that were mock- and AS-ON-treated. Note the dramatic decrease in junctional conductance in cell pairs exposed to the two antisense oligonucleotides (3.0 μM for 48 h). *P < 0.05 (one-way analysis of variance [ANOVA], followed by Student-Newman-Keuls test). Inset: Junctional current responses to transjunctional voltages of 100 mV with abscissa representing the conversion to conductance units; peak responses for mock, AS1-, and AS2-treated cells are 6.5, 0.7, and 2.5 nS, respectively. B: Mean values of the relative changes in Cx43 expression levels after 48 h exposure of confluent cultures of cortical astrocytes to 3.0 μM AS1 and AS2. Cx43 expression levels were normalized against control (untreated cultures: first bar); mean values and standard errors were obtained from three independent experiments; *P < 0.05 (one-way ANOVA, followed by Student-Newman-Keuls test). An example of Cx43 immunoblot is shown on the top of the bar histogram; the arrows indicate the phosphorylated (top two arrows) and the nonphosphorylated (bottom arrow) slates of Cx43; quantification was performed comparing total Cx43 expression within each group.
Fig. 3
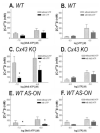
Dose-response curves obtained for 2-MeS-ATP (A,C,E) and UTP (B,D,F) measured in Fura-2-AM-loaded mock-treated wild-type (WT) (A,B) and Cx43 KO (C,D), and anti-sense oligonucleotide (AS-ON)-treated WT (E,F) spinal cord astrocytes. Note that the EC50 values obtained for 2-MeS-ATP are significantly higher in Cx43 knockout (KO) and AS-ON-treated (247 nM and 183 nM, respectively) than in mock-treated WT astrocytes (65 nM). No significant changes in the EC50 values for UTP were observed. Each point in the graphs represents the mean values and standard errors obtained for 10 different litters of WT and Cx43 KO mice and for five independent experiments on AS-treated WT spinal cord astrocytes. The shaded bars on each graph indicate the 95% confidence interval of the EC50 values obtained by nonlinear curve fitting using GraphPad, Prism 3.0 software.
Fig. 4
Changes in functional expression of the adenine-sensitive P2Y1 receptor subtype by downregulation of Cx43 expression levels. Changes in intracellular Ca2+ levels measured in wild-type (WT) (A,B), Cx43 KO (C,D), and antisense oligonucleotide (AS-ON)-treated (E,F) spinal cord astrocytes induced by 0.1 and 1.0 μM 2 MeS-ATP (A,C,E) in the absence (dashed bars) and presence (black bars) of 100 μM UTP to desensitize the uridine-sensitive P2Y2/4 receptors and by 1.0 and 10 μM UTP (B,D,F) in the absence (dashed bars) and presence (black bars) of 100 μM 2-MeS-ATP to desensitize the adenine-sensitive P2Y1 receptors. Note that the amplitudes of Ca2+ responses to 2-MeS-ATP were greatly attenuated in Cx43 KO and AS-ON-treated cells that were pre-incubated with UTP and that no significant changes in Ca2+ amplitudes induced by UTP were measured in these cells when pre-treated with 2-MeS-ATP. Bar histograms represent the mean values and standard errors of three to six independent experiments. *P < 0.001 (one-way analysis of variance [ANOVA], followed by Tukey test).
Fig. 5
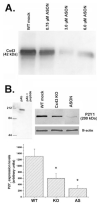
Decreased expression levels of P2Y1 receptors in spinal cord astrocytes after Cx43 downregulation. A: Representative Cx43 immunoblot obtained from whole cell homogenate of confluent cultures of spinal cord astrocytes treated for 48 h with indicated concentrations of oligonucleotides antisense to the mRNA corresponding to exon1 of Cx43 DNA. Note that 3.0 μM was the most effective concentration of AS1 to reduce Cx43 expression levels. B: Upper portion (left) shows immunoblot demonstrating that preabsortion of P2Y1 antibody (pAb) with immunogenic peptide abolishes the 200-kDa band; upper right shows an immunoblot with P2Y1 and β-actin antibodies under different conditions. Bar histogram (below) representing the mean values and standard errors of three to four independent experiments obtained after densitometric analysis of P2Y1 immunoblots obtained from whole cell lysates of mock-treated wild-type (WT), Cx43 knockout (KO), and antisense 1 (AS1)-treated (3.0 μM for 48 h) spinal cord astrocytes. Note that in Cx43 KO and AS1-treated astrocytes P2Y1 expression levels were decreased by about 55% and 75%, respectively. *P < 0.05 (One-way ANOVA, followed by Student-Newman-Keuls test).
Fig. 6
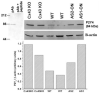
Increased expression levels of P2Y4 receptors in spinal cord astrocytes after downregulation of Cx43. Upper portion (left) shows immunoblot demonstrating that preabsortion of P2Y4 antibody (pAb) with immunogenic peptide abolishes the 84-kDa band. Bar histogram below represents the relative densitometric values (in arbitrary units) obtained from Scion-NIH Image software for the immunoblot performed using P2Y4 and β-actin antibodies (see example at left side of inset). Note the increased expression of P2Y4 receptor in whole cell lysates from Cx43 KO and antisense 1 (AS1)-, AS2-oligonucleotide (ON)-treated cells when compared with wild-type (WT) astrocytes. Cell cultures were obtained from two different litters. Anti-β-actin monoclonal antibody (Sigma) was diluted 1:5,000 and the anti-P2Y4 polyclonal antibody (Alomone) diluted 1:200.
Fig. 7
Properties of intercellular calcium waves propagating between wild-type (WT) (black bars), Cx43 knockout (KO) (white bars), and antisense 1 (AS1)-treated (dashed bars) spinal cord astrocytes when bathed in phosphate-buffered saline (PBS), 3 mM heptanol, and 100 μM of the P2 receptor antagonist PPADS. The bar histograms represent the mean values and standard errors of independent experiments obtained for the efficacy, velocity, and amplitude (EVA) of calcium wave spread (from top to bottom); the overall property of calcium waves, measured by the EVA factor is also displayed. Note that for both Cx43 KO and WT AS1-treated spinal cord cultures, the P2 antagonist (PPADS) was more effective than the gap junction channel blocker, heptanol, in reducing intercellular Ca2+ signaling, while for WT cells, heptanol was more effective than PPADS in reducing wave spread. *P < 0.05 (one-way analysis of variance [ANOVA], followed by Tukey).
Similar articles
- Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment.
Walrave L, Vinken M, Leybaert L, Smolders I. Walrave L, et al. Biomolecules. 2020 Nov 20;10(11):1578. doi: 10.3390/biom10111578. Biomolecules. 2020. PMID: 33233647 Free PMC article. Review. - Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation.
Scemes E, Suadicani SO, Spray DC. Scemes E, et al. J Neurosci. 2000 Feb 15;20(4):1435-45. doi: 10.1523/JNEUROSCI.20-04-01435.2000. J Neurosci. 2000. PMID: 10662834 Free PMC article. - Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation.
Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Suadicani SO, et al. Glia. 2004 Nov 15;48(3):217-29. doi: 10.1002/glia.20071. Glia. 2004. PMID: 15390120 Free PMC article. - The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells.
Henriksen Z, Hiken JF, Steinberg TH, Jørgensen NR. Henriksen Z, et al. Cell Calcium. 2006 May;39(5):435-44. doi: 10.1016/j.ceca.2006.01.012. Epub 2006 Mar 20. Cell Calcium. 2006. PMID: 16545868 - The role of connexin43 in neuropathic pain induced by spinal cord injury.
Wang A, Xu C. Wang A, et al. Acta Biochim Biophys Sin (Shanghai). 2019 Jun 20;51(6):555-561. doi: 10.1093/abbs/gmz038. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31056639 Review.
Cited by
- Astroglial Cell-to-Cell Interaction with Autoreactive Immune Cells in Experimental Autoimmune Encephalomyelitis Involves P2X7 Receptor, β3-Integrin, and Connexin-43.
Milicevic KD, Bataveljic DB, Bogdanovic Pristov JJ, Andjus PR, Nikolic LM. Milicevic KD, et al. Cells. 2023 Jul 5;12(13):1786. doi: 10.3390/cells12131786. Cells. 2023. PMID: 37443820 Free PMC article. - The activation of dormant ependymal cells following spinal cord injury.
Rodriguez-Jimenez FJ, Jendelova P, Erceg S. Rodriguez-Jimenez FJ, et al. Stem Cell Res Ther. 2023 Jul 5;14(1):175. doi: 10.1186/s13287-023-03395-4. Stem Cell Res Ther. 2023. PMID: 37408068 Free PMC article. Review. - Role of Impaired Astrocyte Gap Junction Coupling in Epileptogenesis.
Bedner P, Steinhäuser C. Bedner P, et al. Cells. 2023 Jun 20;12(12):1669. doi: 10.3390/cells12121669. Cells. 2023. PMID: 37371139 Free PMC article. Review. - Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment.
Walrave L, Vinken M, Leybaert L, Smolders I. Walrave L, et al. Biomolecules. 2020 Nov 20;10(11):1578. doi: 10.3390/biom10111578. Biomolecules. 2020. PMID: 33233647 Free PMC article. Review. - Extracellular gentamicin reduces the activity of connexin hemichannels and interferes with purinergic Ca(2+) signaling in HeLa cells.
Figueroa VA, Retamal MA, Cea LA, Salas JD, Vargas AA, Verdugo CA, Jara O, Martínez AD, Sáez JC. Figueroa VA, et al. Front Cell Neurosci. 2014 Sep 4;8:265. doi: 10.3389/fncel.2014.00265. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25237294 Free PMC article.
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998b;10:2129–2142. - PubMed
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-41282/NS/NINDS NIH HHS/United States
- R01 NS041282/NS/NINDS NIH HHS/United States
- NS-41023/NS/NINDS NIH HHS/United States
- R01 NS041023/NS/NINDS NIH HHS/United States
- R01 NS041023-04/NS/NINDS NIH HHS/United States
- R01 NS041023-03/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous