Cell cycle regulators in the neuronal death pathway of amyotrophic lateral sclerosis caused by mutant superoxide dismutase 1 - PubMed (original) (raw)
Cell cycle regulators in the neuronal death pathway of amyotrophic lateral sclerosis caused by mutant superoxide dismutase 1
Minh Dang Nguyen et al. J Neurosci. 2003.
Abstract
There is growing evidence for involvement of members of the cyclin-dependent kinase (Cdk) family in neurodegenerative disorders and in apoptotic death of neurons subjected to various insults. After our recent report that a deregulation of Cdk5 activity by p25 may contribute to pathogenesis of amyotrophic lateral sclerosis (ALS), we further examined the possible involvement of other Cdks in mice expressing a mutant form of superoxide dismutase (SOD1(G37R)) linked to ALS. No substantial changes in Cdk2 or Cdk6 distribution and kinase activities were detected in spinal motor neurons from SOD1(G37R) mice when compared with normal mice. Of particular interest was the upregulation and mislocalization of Cdk4, a regulator of the G1-S checkpoint of the cell cycle, in motor neurons of SOD1(G37R) mice. The increase of Cdk4 activity in SOD1(G37R) mice was associated with an increase in nuclear Cdk4, cyclin D1, its coactivator, and with the abnormal phosphorylation of the retinoblastoma (Rb) protein at Cdk phosphorylation sites. Pharmacological treatment of SOD1(G37R) mice with minocycline, a compound that attenuates microgliosis and slows down disease, lessened the dysregulation of Cdk5/Cdk4 and the phosphorylation of Rb. Interestingly, phospho-Rb was immunoprecipitated with anti-Cdk4 but not with anti-Cdk5 antibodies, suggesting a key role for Cdk4 in the phosphorylation of Rb. Remarkably, the overexpression of a transgene coding for human neurofilament H, a phosphorylation sink for deregulated Cdk5 activity by p25, resulted in a reduction in levels of nuclear Cdk4 and Rb phosphorylation. These results indicate that a cell cycle signaling at the neuronal G1-S checkpoint subsequent to Cdk5 deregulation may constitute a critical step of the neuronal death pathway in ALS caused by mutant SOD1.
Figures
Fig. 1.
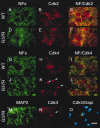
Immunohistochemical staining for Cdks and phospho-pRb in the spinal cord of normal and SOD1G37R mice. Antibodies against Cdk5, Cdk4, Cdk6, and Cdk2 yielded diffuse immunostaining in spinal cord sections of normal mice (A, C, E, G). In contrast, strong immunostaining was obtained for Cdk5 and Cdk4 especially in nuclei of motor neurons from SOD1G37R mice (B, D, white arrows). A high-magnification micrograph at the left corner of D shows a Cdk4-positive nucleus in a motor neuron. Cdk6 immunostaining was enhanced in subsets of motor neurons from SOD1G37R mice (F, white arrows), whereas Cdk2 immunoreactivities were detected in cell bodies of motor neurons (H, white arrows) and in glial cells (H, white arrowheads) of SOD1G37R mice. PSTAIRE antibodies recognizing Cdk1–Cdk3 stained only glial cells in the spinal cord of SOD1G37R mice (J, white arrowheads) but not normal mice (I). Phosphorylated Rb was detected in the nucleus of spinal motor neurons in normal and SOD1G37R mice with phospho-Ser-795-Rb antibodies (K, L). However, the antibodies against phospho-Ser-795-Rb and phospho-Ser-807/Thr-811-Rb detected phosphorylated pRb in cytoplasm of spinal motor neurons exclusively in SOD1G37R mice (L–N, white arrows). Note that two to five neurons per slice from end-stage glutaraldehyde-perfused SOD1 mice exhibit staining for nuclear Cdk4 or phospho-Ser-807/Thr-811-Rb. Western blot analysis revealed 110 kDa phospho-Ser-807/Thr-811-Rb immunoreactivities only in samples from SOD1G37R mice but not in normal mice (O). In contrast, phospho-Ser-795 antibodies recognized 110 kDa phospho species of Rb in both SOD1G37R and normal samples (O). These immunoreactivities compatible with the immunostaining experiments can be abolished with alkaline phosphatase treatment, indicating specificity for both phospho-Rb antibodies. The spinal cord sections were obtained from four SOD1G37R mice (line 29) at the end stage of disease (11–12 months of age) and four normal littermates. Scale bar, 30 μm. The spinal cord lysates were obtained from five SOD1G37R mice (line 29) at the end stage of disease (11–12 months of age) and three normal littermates. All experiments were repeated more than two times.
Fig. 2.
Mislocalization of Cdk4 but not Cdk2 in spinal motor neurons of SOD1G37R mice. Double-immunofluorescence staining confirmed the mislocalization of Cdk4 but not Cdk2 in the nucleus of spinal motor neurons in SOD1G37R mice. Cdk2 was detected in the cytoplasm and nucleolus of spinal motor neurons in normal (WT;A–C) and SOD1G37R(D–F) mice, whereas Cdk4 was found in neuronal cell bodies from normal mice (G–I) and neuronal nuclei from SOD1G37R mice (J–L). RT-97 antibodies were used to stain dendritic and axonal phospho-NF-H, thereby delimiting the neuronal cell bodies. The nuclear localization of Cdk4 in spinal motor neurons of SOD1G37R mice was confirmed by double immunostaining with Cdk4 and MAP2 antibodies and DAPI staining (M–O). A secondary FITC-labeled antibody and a secondary rhodamine-labeled antibody were used to detect phospho-NF-H or MAP-2 and Cdk2 or Cdk4, respectively. Experiments were performed with spinal cord sections or spinal cord lysates from five SOD1G37R mice (line 29) at the end stage of disease (11–12 months old) and four normal littermates. Scale bar, 20 μm. All experiments were repeated more than two times.
Fig. 3.
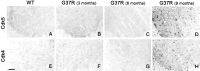
Concomitant dysregulation of Cdk5 and Cdk4 in the late stage of disease in SOD1G37R mice. Antibodies against Cdk5 and Cdk4 yielded diffuse immunostaining in spinal cord sections of normal mice (A, E) and 3- and 6-month-old SOD1G37R mice (B, F, C, G, respectively). In contrast, strong immunostaining for Cdk5 and Cdk4, indicative of dysregulation, starts at 8–9 months of age in SOD1G37R mice (D, H). Experiments were performed with spinal cord sections from three SOD1G37R mice (line 29) at each age and three normal littermates and were repeated more than two times.
Fig. 4.
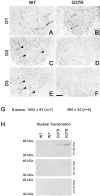
Increase in nuclear cyclin D1 in the spinal cord of SOD1G37R mice. Monoclonal antibodies against cyclin D1 yielded strong nuclear immunoreactivity in the ventral horn of the spinal cord from normal mice (WT) and SOD1G37R mice (A, B), whereas antibodies against cyclins D2 and D3 stained cell bodies of spinal motor neurons (C, E). The weak immunostaining for cyclins D2 and D3 at the end stage of disease in SOD1G37R mice (D, F) likely reflects massive neuronal loss. G, Axonal counts of L5 ventral roots in WT and SOD1G37R mice. To determine whether an increase in Cdk4 activity is associated with increased nuclear levels of cyclin D1, we performed nuclear fractionation. Western blots show that cyclin D1 but not cyclin D2 and D3 levels increase in the nucleus of cells from mutant SOD1 but not WT mice (H). Experiments were performed with spinal cord lysates and sections from four SOD1G37Rmice (line 29) at the end stage of disease (11–12 months old) and four normal littermates and were repeated more than two times. Scale bar, 0.25 mm.
Fig. 5.
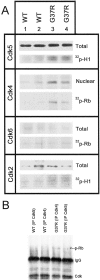
Upregulation of levels and kinase activity of Cdk4 associated with Rb phosphorylation in the spinal cord of SOD1G37R mice. A, Similar levels of Cdk5, Cdk6, and Cdk2 were detected by Western blotting from spinal cord extracts of normal mice (WT) and SOD1G37R mice. However, increased levels of Cdk4 were detected in nuclear fractions from the spinal cord of SOD1G37R mice when compared with normal mice. Kinase assays were performed after immunoprecipitation of spinal cord extracts with Cdk5, Cdk4, Cdk6, and Cdk2 antibodies. The autoradiograms show the quantity of 32P incorporated in histone H1 (Cdk5, Cdk2) or Rb (Cdk4, Cdk6) in spinal cord extracts from SOD1G37R mice and littermates (WT) at 11–12 months of age. The Cdk5 and Cdk4 kinase activities were increased by approximately twofold in samples from SOD1G37R mice, whereas no major changes in Cdk6 and Cdk2 activities were detected. B, Cdk4 but not Cdk5 can be immunoprecipitated with phospho-Rb from spinal cord lysates of SOD1G37R but not WT mice. The membranes were reprobed with antibodies against Cdk5, Cdk4, or phospho-Rb.IP, Immunoprecipitation. Experiments were performed with spinal cord lysates from four SOD1G37R mice (line 29) at the end stage of disease (11–12 months old) and four normal littermates and were repeated more than two times.
Fig. 6.
Attenuation of disease in SOD1G37R mice by minocycline treatment or by overexpression of the hNF-H transgene lessens dysregulation of Cdk4 and Rb phosphorylation. Antibodies against Cdk5 yielded strong immunoreactivities in cell bodies and nuclei of motor neurons in early presymptomatic 10.5-month-old SOD1G37R mice (B). The same mouse also exhibited immunoreactivities for Cdk4 in nuclei of some motor neurons (E, white arrows) as well as activation of microglia (H). Microglia activation, Cdk5 deregulation, and Cdk4 upregulation were reduced in motor neurons of 10.5-month-old SOD1G37R mice treated with minocycline at 9 months (C, F, I). Similar staining intensities were found in normal littermates (A, D, G). Less phosphorylation of Rb was detected in SOD1G37R mice as a result of minocycline treatment or hNF-H overexpression (N, O). Double immunohistochemical staining for Cdk4 and activated caspase-3 on spinal cord sections from SOD1G37R mice indicates rare neurons positive for both Cdk4 and activated caspase-3 (P), yet few of them were positive for both antibodies (Q). This finding suggests that a deregulation of Cdk4 may be independent of other neuronal death pathways involving caspase-3, that apoptotic processes in ALS mice differ from the one occurring during neuronal development, or both. The experiments were performed with spinal cord sections from five SOD1G37R mice (line 29) at the end stage of disease (11–12 months old), two SOD1G37R;hNF-H mice (12 months old), and four normal littermates. Scale bar, 50 μm.
Fig. 7.
Overexpression of hNF-H in SOD1G37R mice reduces Cdk4 levels. Antibodies against Cdk5 yielded strong immunoreactivities for cell bodies and nuclei of motor neurons in 13-month-old SOD1G37Rmice overexpressing hNF-H (A, white arrows). However, the same mouse exhibited poor immunoreactivities for Cdk4 in nuclei of motor neurons (B, white arrows) except for one neuron (B, black arrow). Similar levels of Cdk5 were detected by Western blotting in spinal cord extracts of normal mice (WT) and SOD1G37R mice with or without the hNF-H transgene (lanes 1–6). In contrast, the overexpression of hNF-H decreased the total levels of Cdk4 in the spinal cord of SOD1G37R mice (lanes 5, 6). Actin was used as a control. Experiments were performed with spinal cord sections from three 12- to 13-month-old SOD1G37R;hNF-H mice. The spinal cord samples in D were prepared from three 12-month-old mice of each genotype. All experiments were repeated more than twice.
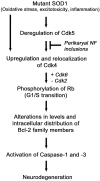
Fig. 8.
Model of the neuronal death pathway involving Cdks. In this model, mutant SOD1, oxidative stress, excitotoxicity, and inflammation can trigger deregulation of Cdk5 that subsequently upregulates Cdk4. The activation of nuclear Cdk4 leads to the phosphorylation of Rb, promoting its dissociation from E2F-1 with ensuing transcription of proapoptotic Bcl-2 family members. This provokes mitochondrial disturbances, caspase activation, and ultimately neurodegeneration. The toxicity of nuclear Cdk4 upregulation, Rb phosphorylation, and the ensuing signaling pathway can be attenuated by interference of deregulated Cdk5 activity. For instance, this may be achieved by overexpressing NF-H, which acts as a phosphorylation sink for deregulated Cdk5 activity. Cdk4 can also be activated independently of Cdk5 deregulation.
Similar articles
- Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions.
Nguyen MD, Larivière RC, Julien JP. Nguyen MD, et al. Neuron. 2001 Apr;30(1):135-47. doi: 10.1016/s0896-6273(01)00268-9. Neuron. 2001. PMID: 11343650 - Mutant superoxide dismutase 1 causes motor neuron degeneration independent of cyclin-dependent kinase 5 activation by p35 or p25.
Takahashi S, Kulkarni AB. Takahashi S, et al. J Neurochem. 2004 Mar;88(5):1295-304. doi: 10.1046/j.1471-4159.2003.02256.x. J Neurochem. 2004. PMID: 15009685 - Cdk5 sinks into ALS.
Patzke H, Tsai LH. Patzke H, et al. Trends Neurosci. 2002 Jan;25(1):8-10. doi: 10.1016/s0166-2236(00)02000-2. Trends Neurosci. 2002. PMID: 11801324 Review. - Cyclin-dependent kinase-5 (CDK5) and amyotrophic lateral sclerosis.
Bajaj NP. Bajaj NP. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Dec;1(5):319-27. doi: 10.1080/146608200300079563. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000. PMID: 11464850 Review.
Cited by
- Delayed cell cycle pathway modulation facilitates recovery after spinal cord injury.
Wu J, Stoica BA, Dinizo M, Pajoohesh-Ganji A, Piao C, Faden AI. Wu J, et al. Cell Cycle. 2012 May 1;11(9):1782-95. doi: 10.4161/cc.20153. Epub 2012 May 1. Cell Cycle. 2012. PMID: 22510563 Free PMC article. - Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS.
Ferraiuolo L, Heath PR, Holden H, Kasher P, Kirby J, Shaw PJ. Ferraiuolo L, et al. J Neurosci. 2007 Aug 22;27(34):9201-19. doi: 10.1523/JNEUROSCI.1470-07.2007. J Neurosci. 2007. PMID: 17715356 Free PMC article. - CDDO-Me Selectively Attenuates CA1 Neuronal Death Induced by Status Epilepticus via Facilitating Mitochondrial Fission Independent of LONP1.
Kim JE, Park H, Choi SH, Kong MJ, Kang TC. Kim JE, et al. Cells. 2019 Aug 5;8(8):833. doi: 10.3390/cells8080833. Cells. 2019. PMID: 31387295 Free PMC article. - Wild-Type and Mutant FUS Expression Reduce Proliferation and Neuronal Differentiation Properties of Neural Stem Progenitor Cells.
Stronati E, Biagioni S, Fiore M, Giorgi M, Poiana G, Toselli C, Cacci E. Stronati E, et al. Int J Mol Sci. 2021 Jul 15;22(14):7566. doi: 10.3390/ijms22147566. Int J Mol Sci. 2021. PMID: 34299185 Free PMC article. - An in vitro Model of Human Retinal Detachment Reveals Successive Death Pathway Activations.
Potic J, Mbefo M, Berger A, Nicolas M, Wanner D, Kostic C, Matet A, Behar-Cohen F, Moulin A, Arsenijevic Y. Potic J, et al. Front Neurosci. 2020 Nov 26;14:571293. doi: 10.3389/fnins.2020.571293. eCollection 2020. Front Neurosci. 2020. PMID: 33324144 Free PMC article.
References
- Abi-Farah CA, Nguyen MD, Julien JP, Leclerc N. Alterations in microtubule associated proteins before disease onset in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2002;84:77–86. - PubMed
- Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease. NeuroReport. 1996;7:3047–3049. - PubMed
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Rheaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous