Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex - PubMed (original) (raw)
Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex
Stan F J van de Graaf et al. EMBO J. 2003.
Abstract
TRPV5 and TRPV6 constitute the Ca(2+) influx pathway in a variety of epithelial cells. Here, we identified S100A10 as the first auxiliary protein of these epithelial Ca(2+) channels using yeast two-hybrid and GST pull-down assays. This S100 protein forms a heterotetrameric complex with annexin 2 and associates specifically with the conserved sequence VATTV located in the C-terminal tail of TRPV5 and TRPV6. Of these five amino acids, the first threonine plays a crucial role since the corresponding mutants (TRPV5 T599A and TRPV6 T600A) exhibited a diminished capacity to bind S100A10, were redistributed to a subplasma membrane area and did not display channel activity. Using GST pull-down and co-immunoprecipitation assays we demonstrated that annexin 2 is part of the TRPV5-S100A10 complex. Furthermore, the S100A10-annexin 2 pair colocalizes with the Ca(2+) channels in TRPV5-expressing renal tubules and TRPV6-expressing duodenal cells. Importantly, downregulation of annexin 2 using annexin 2-specific small interfering RNA inhibited TRPV5 and TRPV6-mediated currents in transfected HEK293 cells. In conclusion, the S100A10-annexin 2 complex plays a crucial role in routing of TRPV5 and TRPV6 to plasma membrane.
Figures
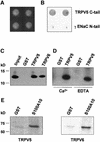
Fig. 1. Interaction of TRPV5 and S100A10 as shown by yeast two- hybrid and GST pull-down analyses. (A) The C-terminal tail of TRPV5 or γENaC and full-length S100A10 were cotransformed into the Y153 yeast strain and grown on media without tryptophan and leucine. (B) β-galactosidase activity was demonstrated in TRPV5 and S100A10 cotransformed yeast, whereas no activity was observed in γENaC and S100A10 cotransformed yeast. Two representative colonies are depicted. (C) Lysates of X.laevis oocytes injected with 20 ng of VSV-tagged S100A10 cRNA were incubated with GST or GST fused to the C-terminal tail of TRPV5 or TRPV6 immobilized on glutathione– Sepharose 4B beads. S100A10 interacted specifically with TRPV5 and TRPV6, but not with GST alone. (D) The experiment was performed as outlined in (C). Binding of S100A10 to TRPV5 was demonstrated in the presence of 1 mM Ca2+ or 2 mM EDTA. (E) [35S]methionine- labeled full-length TRPV5 or TRPV6 was incubated with GST or GST–S100A10 immobilized on glutathione–Sepharose 4B beads. Both TRPV5 and TRPV6 interacted with S100A10, whereas no binding to GST alone was observed.
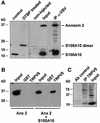
Fig. 2. Annexin 2 interacts with TRPV5 via S100A10. Xenopus laevis oocytes were injected with S100A10 or co-injected with annexin 2 and VSV-tagged S100A10 cRNAs. (A) Lysates of S100A10 cRNA-injected oocytes were treated with DTBP and analyzed by immunoblot. The chemically cross-linked S100A10 band runs at 23 kDa, exactly the expected size of a VSV-tagged S100A10 dimer. Homogenates of non-injected and oocytes co-injected with S100A10 and annexin 2 cRNAs were subjected to immunoprecipitation using monoclonal anti-VSV antibodies. Annexin 2 co-immunoprecipitated with S100A10 as was visualized by autoradiography of the metabolically labeled proteins. As a control, the expression of S100A10 and annexin 2 in the co-injected oocytes was demonstrated by immunoblot analysis to demonstrate that the precipitated proteins were of the correct size. (B) Homogenates of annexin 2 cRNA-injected or S100A10 and annexin 2 cRNA-co-injected oocytes were incubated with GST alone or GST fusion protein containing the TRPV5 C-terminal tail immobilized on glutathione–Sepharose 4B beads. The association of annexin 2 with TRPV5 in the presence of S100A10 was demonstrated by immunoblot using a monoclonal anti-annexin 2 antibody. (C) Full-length annexin 2, S100A10 and TRPV5 were in vitro translated using a reticulocyte lysate system in the presence of canine microsomal membranes. S100A10 and annexin 2 were co-immunoprecipitated with TRPV5 confirming the formation of a TRPV5–S100A10–annexin 2 complex.
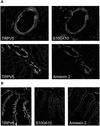
Fig. 3. Colocalization of TRPV5 and TRPV6 with S100A10 and annexin 2. (A) Kidney sections were costained with antibodies against TRPV5 and S100A10 or TRPV5 and annexin 2. (B) Duodenum sections were stained with antibodies against TRPV6, S100A10 and annexin 2. See Supplementary data for color images of this figure.
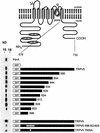
Fig. 4. Mapping of the S100A10 binding site in TRPV5. GST fusion proteins containing different portions of the C-terminal tail of TRPV5 were constructed according to the schematic drawing. These proteins were immobilized on glutathione–Sepharose 4B beads and then incu bated with lysates from X.laevis oocytes injected with 20 ng of S100A10 cRNA. Interaction of S100A10 with the GST fusion proteins was determined by immunoblotting. The binding site was localized between amino acids 598 and 603. Virtually all interaction with S100A10 was abolished when this region (VATTV) was mutated into glycines. A similar effect was observed with the single point mutant TRPV5 T600A.
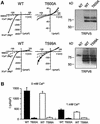
Fig. 5. Activity and subcellular localization of TRPV5 and TRPV6 with a mutated S100A10 binding site. HEK293 cells were transiently transfected with TRPV5 (wild type or T600A) or TRPV6 (wild type or T599A) and analyzed using the whole-cell patch–clamp configuration and immunoblotting. (A) Currents were measured during voltage ramps from –150 to +100 mV (400 ms) in the absence of permeable cations (all substituted by NMDG+), in the absence of divalent cations only, and in the presence of 1 mM Ca2+. The I/V curves showed inward rectification typical for TRPV5 and TRPV6. In the TRPV5 T600A and TRPV6 T599A mutants Na+ and Ca2+ currents were virtually abolished, while the channel was readily detectable. NT, not transfected. (B) The average currents measured in the absence of divalent cations and in the presence of 1 mM Ca2+ of three independent transfections for wild-type and mutant TRPV5 and TRPV6 are depicted. (C) Immunocytochemistry was performed on X.laevis oocytes injected with 5 ng of HA-tagged TRPV5 (wild type or T600A) or Flag-tagged TRPV6 (wild type or T599A) cRNA. Oocytes injected with wild-type channels showed predominant immunopositive staining at the plasma membrane, whereas the channels containing a mutation in the S100A10 binding site accumulated in an area just below the plasma membrane. Representative images of three independent experiments are shown.
Fig. 5. Activity and subcellular localization of TRPV5 and TRPV6 with a mutated S100A10 binding site. HEK293 cells were transiently transfected with TRPV5 (wild type or T600A) or TRPV6 (wild type or T599A) and analyzed using the whole-cell patch–clamp configuration and immunoblotting. (A) Currents were measured during voltage ramps from –150 to +100 mV (400 ms) in the absence of permeable cations (all substituted by NMDG+), in the absence of divalent cations only, and in the presence of 1 mM Ca2+. The I/V curves showed inward rectification typical for TRPV5 and TRPV6. In the TRPV5 T600A and TRPV6 T599A mutants Na+ and Ca2+ currents were virtually abolished, while the channel was readily detectable. NT, not transfected. (B) The average currents measured in the absence of divalent cations and in the presence of 1 mM Ca2+ of three independent transfections for wild-type and mutant TRPV5 and TRPV6 are depicted. (C) Immunocytochemistry was performed on X.laevis oocytes injected with 5 ng of HA-tagged TRPV5 (wild type or T600A) or Flag-tagged TRPV6 (wild type or T599A) cRNA. Oocytes injected with wild-type channels showed predominant immunopositive staining at the plasma membrane, whereas the channels containing a mutation in the S100A10 binding site accumulated in an area just below the plasma membrane. Representative images of three independent experiments are shown.
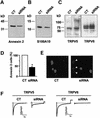
Fig. 6. SiRNA-mediated annexin 2 gene silencing inhibits channel activity in HEK293 cells heterologously expressing TRPV5. Immunoblot showing annexin 2 (A), S100A10 (B), TRPV5 and TRPV6 (C) protein levels in control (CT) and annexin 2-specific siRNA-treated HEK293 cells. Images (E) and corresponding histogram (D) of annexin 2 immunofluorescence staining of control and annexin 2-specific siRNA transfected HEK293 cells. Ca2+ currents in response to a voltage step from +20 to –100 mV in control and annexin 2-specific siRNA transfected HEK293 cells (F). Current response to a voltage ramp from –150 to 100 mV measured in NMDG+ and divalent-free solution in control and annexin 2-specific siRNA transfected HEK293 cells (G). Distribution of Na+ currents as a measure of channel activity analyzed in control (open bars) and annexin 2-specific siRNA-transfected (closed bars) HEK293 cells at –80 mV (H) and the corresponding averaged data (I). N is more than 25 cells of two independent transfections. *P < 0.001 significantly different from control. CT, control.
Fig. 6. SiRNA-mediated annexin 2 gene silencing inhibits channel activity in HEK293 cells heterologously expressing TRPV5. Immunoblot showing annexin 2 (A), S100A10 (B), TRPV5 and TRPV6 (C) protein levels in control (CT) and annexin 2-specific siRNA-treated HEK293 cells. Images (E) and corresponding histogram (D) of annexin 2 immunofluorescence staining of control and annexin 2-specific siRNA transfected HEK293 cells. Ca2+ currents in response to a voltage step from +20 to –100 mV in control and annexin 2-specific siRNA transfected HEK293 cells (F). Current response to a voltage ramp from –150 to 100 mV measured in NMDG+ and divalent-free solution in control and annexin 2-specific siRNA transfected HEK293 cells (G). Distribution of Na+ currents as a measure of channel activity analyzed in control (open bars) and annexin 2-specific siRNA-transfected (closed bars) HEK293 cells at –80 mV (H) and the corresponding averaged data (I). N is more than 25 cells of two independent transfections. *P < 0.001 significantly different from control. CT, control.
Similar articles
- The annexin 2-S100A10 complex and its association with TRPV6 is regulated by cAMP/PKA/CnA in airway and gut epithelia.
Borthwick LA, Neal A, Hobson L, Gerke V, Robson L, Muimo R. Borthwick LA, et al. Cell Calcium. 2008 Aug;44(2):147-57. doi: 10.1016/j.ceca.2007.11.001. Epub 2008 Jan 9. Cell Calcium. 2008. PMID: 18187190 - Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1alpha,25-dihydroxy Vitamin D3 and dietary Ca2+.
van de Graaf SF, Boullart I, Hoenderop JG, Bindels RJ. van de Graaf SF, et al. J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):303-8. doi: 10.1016/j.jsbmb.2004.03.029. J Steroid Biochem Mol Biol. 2004. PMID: 15225790 Review. - Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6.
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Hoenderop JG, et al. EMBO J. 2003 Feb 17;22(4):776-85. doi: 10.1093/emboj/cdg080. EMBO J. 2003. PMID: 12574114 Free PMC article. - Regulation of CFTR function by annexin A2-S100A10 complex in health and disease.
Muimo R. Muimo R. Gen Physiol Biophys. 2009;28 Spec No Focus:F14-9. Gen Physiol Biophys. 2009. PMID: 20093721 Review. - The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6.
Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. Lu P, et al. Nephrol Dial Transplant. 2008 Nov;23(11):3397-402. doi: 10.1093/ndt/gfn291. Epub 2008 May 21. Nephrol Dial Transplant. 2008. PMID: 18495742
Cited by
- PtdIns(4,5)P2 interacts with CaM binding domains on TRPM3 N-terminus.
Holendova B, Grycova L, Jirku M, Teisinger J. Holendova B, et al. Channels (Austin). 2012 Nov-Dec;6(6):479-82. doi: 10.4161/chan.22177. Epub 2012 Sep 18. Channels (Austin). 2012. PMID: 22989896 Free PMC article. - Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis.
Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D. Mirsaeidi M, et al. J Transl Med. 2016 Apr 12;14:89. doi: 10.1186/s12967-016-0843-7. J Transl Med. 2016. PMID: 27071553 Free PMC article. Review. - Regulation of inflammatory response in human chondrocytes by lentiviral mediated RNA interference against S100A10.
Song C, Zhou X, Dong Q, Fan R, Wu G, Ji B, Meng Q, Zheng M. Song C, et al. Inflamm Res. 2012 Nov;61(11):1219-27. doi: 10.1007/s00011-012-0519-6. Epub 2012 Jul 14. Inflamm Res. 2012. PMID: 22797859 - Involvement of Annexin A2 in p53 induced apoptosis in lung cancer.
Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen F, Zhao YZ, Fu SB. Huang Y, et al. Mol Cell Biochem. 2008 Feb;309(1-2):117-23. doi: 10.1007/s11010-007-9649-5. Epub 2007 Nov 16. Mol Cell Biochem. 2008. PMID: 18008140 - Genome-wide scans for loci under selection in humans.
Ronald J, Akey JM. Ronald J, et al. Hum Genomics. 2005 Jun;2(2):113-25. doi: 10.1186/1479-7364-2-2-113. Hum Genomics. 2005. PMID: 16004726 Free PMC article. Review.
References
- Ali S.M., Geisow,M.J. and Burgoyne,R.D. (1989) A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature, 340, 313–315. - PubMed
- Dreier R., Schmid,K.W., Gerke,V. and Riehemann,K. (1998) Differential expression of annexins I, II and IV in human tissues: an immunohistochemical study. Histochem. Cell Biol., 110, 137–148. - PubMed
- Durfee T., Becherer,K., Chen,P.L., Yeh,S.H., Yang,Y., Kilburn,A.E., Lee,W.H. and Elledge,S.J. (1993) The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev., 7, 555–569. - PubMed
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous