Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells - PubMed (original) (raw)
. 2003 Apr 29;100(9):5187-92.
doi: 10.1073/pnas.0931282100. Epub 2003 Apr 16.
Marianne Hoy, Wei Zhang, Alejandro M Bertorello, Krister Bokvist, Kirsten Capito, Alexander M Efanov, Björn Meister, Peter Thams, Shao-Nian Yang, Patrik Rorsman, Per-Olof Berggren, Jesper Gromada
Affiliations
- PMID: 12700357
- PMCID: PMC154320
- DOI: 10.1073/pnas.0931282100
Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells
Hervør L Olsen et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Insulin secretion is controlled by the beta cell's metabolic state, and the ability of the secretory granules to undergo exocytosis increases during glucose stimulation in a membrane potential-independent fashion. Here, we demonstrate that exocytosis of insulin-containing secretory granules depends on phosphatidylinositol 4-kinase (PI 4-kinase) activity and that inhibition of this enzyme suppresses glucose-stimulated insulin secretion. Intracellular application of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P(2)] stimulated exocytosis by promoting the priming of secretory granules for release and increasing the number of granules residing in a readily releasable pool. Reducing the cytoplasmic ADP concentration in a way mimicking the effects of glucose stimulation activated PI 4-kinase and increased exocytosis whereas changes of the ATP concentration in the physiological range had little effect. The PI(4,5)P(2)-binding protein Ca(2+)-dependent activator protein for secretion (CAPS) is present in beta cells, and neutralization of the protein abolished both Ca(2+)- and PI(4,5)P(2)-induced exocytosis. We conclude that ADP-induced changes in PI 4-kinase activity, via generation of PI(4,5)P(2), represents a metabolic sensor in the beta cell by virtue of its capacity to regulate the release competence of the secretory granules.
Figures
Figure 1
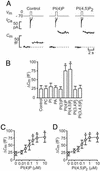
PI(4)P and PI(4,5)P2 stimulate exocytosis in β cells. (A) Individual voltage-clamped mouse β cells were stimulated with single 500-ms depolarizations (_V_m, Top). The resultant Ca2+ currents (_I_Ca, Middle) and increases in cell capacitance (_C_m, reflecting fusion of insulin-containing secretory granules with the plasma membrane) recorded under control conditions and in the presence of 1 μM PI(4)P and 1 μM PI(4,5)P2 are shown. (B) Histogram summarizing the capacitance increases elicited by single voltage-clamp depolarizations from −70 mV to 0 mV under control conditions and after inclusion of 1 μM of phosphatidylcholine (PC), PI, phosphatic acid (PA), PI(4)P, PI(4,5)P2, PI 3,4-bisphosphate [PI(3,4)P2], and PI 3,4,5-trisphosphate [PI(3,4,5)P3]. (C) Capacitance increases (Δ_C_m) elicited by single voltage-clamp depolarizations recorded in the presence of increasing intracellular concentrations (0–10 μM) of PI(4)P. (D) As in C but using PI(4,5)P2 instead. The curves in C and D represent least-squares fit of the mean values to the Hill equation. Data are quoted as mean values ± SEM of five to seven individual experiments. *, P < 0.05.
Figure 2
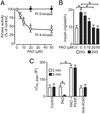
PI(4)P and PI(4,5)P2 are required for refilling of RRP. (A) Individual β cells were stimulated by using a train of fourteen 500-ms depolarizations (_V_m, Upper). The associated increases in cell capacitance (_C_m, Lower) were measured under control conditions and after inclusion of 1 μM PI(4)P in the pipette solution dialyzing the cell interior. (B) Histogram summarizing the magnitude of the exocytotic responses elicited by trains of depolarizations (_C_m, train) under control conditions and in the presence of 1 μM PI(4)P and 1 μM PI(4,5)P2. (C) Exocytosis (_C_m) elicited by two trains of depolarizations 2 min (Left) and 5 min (Right) after establishment of standard whole-cell configuration under control conditions (control) and in a different cell after infusion of an antibody directed against PI(4)P [25 μg/ml, anti-PI(4)P]. (D) Histogram summarizing exocytosis elicited by 1st (2 min, open bars) and 2nd train of depolarizations (5 min after reaching whole-cell configuration, filled bars) under control conditions, in the presence of 25 μg/ml anti-PI(4)P, 25 μg/ml of an antibody directed against PI(4,5)P2 [anti-PI(4,5)P2] or 25 μg/ml of an antibody against phosphatidylserine (anti-PS). In B and D, data are quoted as mean values ± SEM of five experiments in each group. *, P < 0.01.
Figure 3
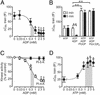
PI 4-kinase activity is required for RRP refilling and insulin secretion in mouse β cells. (A) PI 4-kinase (open circles) and PI 5-kinase activities (filled circles) measured in islet homogenates in the presence of 0–50 μM PAO. (B) Insulin secretion measured during 1 h in the presence of 30 mM extracellular K+ and 0.2 mM diazoxide at 5 mM (open bar) and 20 mM glucose plus 0–50 μM PAO (filled bars). (C) Histogram summarizing exocytosis elicited by first (2 min, open bars) and second train of depolarizations (5 min after reaching whole-cell configuration, filled bars) under control conditions and in the presence of 20 μM PAO with or without 1 μM of PI(4)P and after inclusion of antibody directed against type II PI 4-kinase (anti-4C5G). Data are presented as mean values ± SEM of four (A), five (B), and five (C) experiments. *, P < 0.01.
Figure 4
ADP inhibits exocytosis by suppression of PI 4-kinase activity and RRP replenishment. (A) Exocytosis (Δ_C_m) elicited by the second of two trains of fourteen 500-ms depolarizations applied 2 and 5 min after establishment of standard whole-cell configuration in the presence 0–5 mM Mg-ADP and 3 mM ATP. The responses to the first train were unaffected by the nucleotide. The curve was drawn by approximating the Hill equation to the mean values. The shaded area highlights the physiological range of ADP concentrations observed at low and high glucose (data taken from 30). (B) Histogram summarizing exocytosis elicited by first (2 min, open bars) and second (5 min after establishing whole-cell configuration, filled bars) train of depolarizations under control conditions (3 mM ATP alone), in the presence of 3 mM ATP and 3 mM ADP, in the presence of 3 mM ATP, 3 mM ADP, and 1 μM of either PI(4)P or PI(4,5)P2. (C) PI 4-kinase (open circles) and PI 5-kinase (filled circles) activities measured in islet homogenates in the presence of 0–5 mM ADP. The curve for PI 4-kinase activity was drawn by approximating the Hill equation to the mean values. The shaded area highlights the physiological range of ADP concentrations observed at low and high glucose (data taken from ref. 30). (D) As in A but the experiment was conducted in the presence of 0–5 mM Mg-ATP. The shaded area indicates the range of ATP concentrations measured in β cells at low and high glucose concentrations (data taken from ref. 30). Data are presented as mean values ± SEM of five experiments. *, P < 0.05; **, P < 0.01.
Figure 5
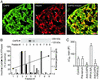
CAPS is involved in Ca2+- and PI(4,5)P2-dependent exocytosis in β cells. (A) Images of sections of rat endocrine pancreas stained for CAPS (Left) and insulin (Center) and after overlay (Right). CAPS immunoreactivity in single β cells is indicated by arrows. (Scale bar = 20 μm.) (B Upper) Western blot illustrating the presence of CAPS in different subcellular fractions 1–9. (B Lower) Quantitation of the abundance of CAPS (filled bars) and Na+, K+-ATPase α-subunit (plasma membrane fractions, open bars), insulin content (granule-enriched fractions, hatched bars), and the sucrose gradient (black line). The experiment was repeated twice with identical results. (C) Histogram summarizing exocytosis elicited by trains of depolarizations applied 2 min after establishment of the whole-cell configuration under control conditions, in the presence of preimmune serum (preserum; 1.5 mg/ml), after inclusion of an antibody against CAPS (anti-CAPS, 1.5 mg/ml), and the CAPS antibody plus 1 μM of PI(4,5)P2. Data are mean values ± SEM of five experiments. *, P < 0.01.
Similar articles
- Neuronal calcium sensor-1 potentiates glucose-dependent exocytosis in pancreatic beta cells through activation of phosphatidylinositol 4-kinase beta.
Gromada J, Bark C, Smidt K, Efanov AM, Janson J, Mandic SA, Webb DL, Zhang W, Meister B, Jeromin A, Berggren PO. Gromada J, et al. Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10303-8. doi: 10.1073/pnas.0504487102. Epub 2005 Jul 12. Proc Natl Acad Sci U S A. 2005. PMID: 16014415 Free PMC article. - Glucose-stimulated signaling pathways in biphasic insulin secretion.
Straub SG, Sharp GW. Straub SG, et al. Diabetes Metab Res Rev. 2002 Nov-Dec;18(6):451-63. doi: 10.1002/dmrr.329. Diabetes Metab Res Rev. 2002. PMID: 12469359 Review. - Rapid ATP-dependent priming of secretory granules precedes Ca(2+)-induced exocytosis in mouse pancreatic B-cells.
Eliasson L, Renström E, Ding WG, Proks P, Rorsman P. Eliasson L, et al. J Physiol. 1997 Sep 1;503 ( Pt 2)(Pt 2):399-412. doi: 10.1111/j.1469-7793.1997.399bh.x. J Physiol. 1997. PMID: 9306281 Free PMC article. - Imidazoline NNC77-0074 stimulates insulin secretion and inhibits glucagon release by control of Ca(2+)-dependent exocytosis in pancreatic alpha- and beta-cells.
Høy M, Olsen HL, Andersen HS, Bokvist K, Buschard K, Hansen J, Jacobsen P, Petersen JS, Rorsman P, Gromada J. Høy M, et al. Eur J Pharmacol. 2003 Apr 11;466(1-2):213-21. doi: 10.1016/s0014-2999(03)01537-1. Eur J Pharmacol. 2003. PMID: 12679159 - Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion.
Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu YJ, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H, Sharp GW. Bratanova-Tochkova TK, et al. Diabetes. 2002 Feb;51 Suppl 1:S83-90. doi: 10.2337/diabetes.51.2007.s83. Diabetes. 2002. PMID: 11815463 Review.
Cited by
- Regulation of insulin secretion by phosphatidylinositol-4,5-bisphosphate.
Tomas A, Yermen B, Regazzi R, Pessin JE, Halban PA. Tomas A, et al. Traffic. 2010 Jan;11(1):123-37. doi: 10.1111/j.1600-0854.2009.00996.x. Traffic. 2010. PMID: 19845918 Free PMC article. - CAPS and Munc13 utilize distinct PIP2-linked mechanisms to promote vesicle exocytosis.
Kabachinski G, Yamaga M, Kielar-Grevstad DM, Bruinsma S, Martin TF. Kabachinski G, et al. Mol Biol Cell. 2014 Feb;25(4):508-21. doi: 10.1091/mbc.E12-11-0829. Epub 2013 Dec 19. Mol Biol Cell. 2014. PMID: 24356451 Free PMC article. - Munc13 homology domain-1 in CAPS/UNC31 mediates SNARE binding required for priming vesicle exocytosis.
Khodthong C, Kabachinski G, James DJ, Martin TF. Khodthong C, et al. Cell Metab. 2011 Aug 3;14(2):254-63. doi: 10.1016/j.cmet.2011.07.002. Cell Metab. 2011. PMID: 21803295 Free PMC article. - Neuronal calcium sensor-1 potentiates glucose-dependent exocytosis in pancreatic beta cells through activation of phosphatidylinositol 4-kinase beta.
Gromada J, Bark C, Smidt K, Efanov AM, Janson J, Mandic SA, Webb DL, Zhang W, Meister B, Jeromin A, Berggren PO. Gromada J, et al. Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10303-8. doi: 10.1073/pnas.0504487102. Epub 2005 Jul 12. Proc Natl Acad Sci U S A. 2005. PMID: 16014415 Free PMC article. - Phosphatidylinositol-4-phosphate signaling regulates dense granule biogenesis and exocytosis in Toxoplasma gondii.
Arabiotorre A, Formanowicz M, Bankaitis VA, Grabon A. Arabiotorre A, et al. bioRxiv [Preprint]. 2023 Jan 9:2023.01.09.523261. doi: 10.1101/2023.01.09.523261. bioRxiv. 2023. PMID: 36712082 Free PMC article. Preprint.
References
- Dean P M. Diabetologia. 1973;9:115–119. - PubMed
- Olofsson C S, Gopel S O, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Pflügers Arch. 2002;444:43–51. - PubMed
- Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. News Physiol Sci. 2000;15:72–77. - PubMed
- Easom R A. Semin Cell Dev Biol. 2000;11:253–266. - PubMed
- Hosker J P, Rudenski A S, Burnett M A, Matthews D R, Turner R C. Metabolism. 1989;38:767–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous