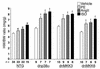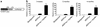Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling - PubMed (original) (raw)
. 2003 May;111(10):1475-86.
doi: 10.1172/JCI17295.
Orlando F Bueno, Qiangrong Liang, Benjamin J Wilkins, Yan-Shan Dai, Stephanie Parsons, Joseph Braunwart, Betty J Glascock, Raisa Klevitsky, Thomas F Kimball, Timothy E Hewett, Jeffery D Molkentin
Affiliations
- PMID: 12750397
- PMCID: PMC155046
- DOI: 10.1172/JCI17295
Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling
Julian C Braz et al. J Clin Invest. 2003 May.
Abstract
The MAPKs are important transducers of growth and stress stimuli in virtually all eukaryotic cell types. In the mammalian heart, MAPK signaling pathways have been hypothesized to regulate myocyte growth in response to developmental signals or physiologic and pathologic stimuli. Here we generated cardiac-specific transgenic mice expressing dominant-negative mutants of p38alpha, MKK3, or MKK6. Remarkably, attenuation of cardiac p38 activity produced a progressive growth response and myopathy in the heart that correlated with the degree of enzymatic inhibition. Moreover, dominant-negative p38alpha, MKK3, and MKK6 transgenic mice each showed enhanced cardiac hypertrophy following aortic banding, Ang II infusion, isoproterenol infusion, or phenylephrine infusion for 14 days. A mechanism underlying this enhanced-growth profile was suggested by the observation that dominant-negative p38alpha directly augmented nuclear factor of activated T cells (NFAT) transcriptional activity and its nuclear translocation. In vivo, NFAT-dependent luciferase reporter transgenic mice showed enhanced activation in the presence of the dominant-negative p38alpha transgene before and after the onset of cardiac hypertrophy. More significantly, genetic disruption of the calcineurin Abeta gene rescued hypertrophic cardiomyopathy and depressed functional capacity observed in p38-inhibited mice. Collectively, these observations indicate that reduced p38 signaling in the heart promotes myocyte growth through a mechanism involving enhanced calcineurin-NFAT signaling.
Figures
Figure 1
Generation of cardiac-specific transgenic mice expressing dominant-negative mutants of p38α, MKK3, and MKK6. (a) Western blot analysis with Ab’s against p38α, p38β, MKK3, and MKK6 from nontransgenic (NTG) and transgenic (TG) hearts regulated by the α-MHC promoter (b) Western blot analysis of p38 phosphorylation in the hearts of nontransgenic (wild-type littermates) or dnMKK3 and dnMKK6 transgenic mice injected for 30 min with either PBS or PE (10 mg/kg). To verify specificity, phospho–ERK-1/2 (phos-ERK-1/2) and phospho-JNK (phos-JNK) were also assayed. The asterisks show the reduced phosphorylation of p38 at baseline (PBS) and in response to PE stimulation. (c) p38 immune kinase assay from PBS- and PE-injected nontransgenic mice or dnp38α, dnMKK3, and dnMKK6 mice. Thirty minutes after stimulation, the hearts were removed and phosphorylation of MBP was monitored by immune kinase assay with p38-specific Ab. Three independent p38 immune kinase assays showed increased activity in NTG hearts and dnMKK6 hearts. Veh, vehicle. (d) Western blot analysis of MAPKAPK2 phosphorylation (phos-MKAPK2), a direct p38 target, in the hearts of nontransgenic (wild-type littermates) or each of the dominant-negative transgenic mice after PE stimulation (10 mg/kg). All three dominant-negative strategies significantly reduced p38 kinase activity in c and d (#P < 0.05 versus NTG vehicle injected; †P < 0.05 versus NTG PE-injected).
Figure 2
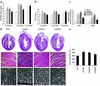
Dnp38α, dnMKK3, and dnMKK6 transgenic mice show progressive cardiac hypertrophy at baseline. (a) Heart-to-body weight ratio (HW/BW) measurements at 2, 4, and 8 months of age show a progressive increase in heart size in dnp38α, dnMKK3, and dnMKK6 transgenic mice compared with nontransgenics. Four animals were assayed at 2 and 4 months, while six animals were measured at 8 months in each group. (b) Measurement of left ventricular diastolic dimension (LVED) by echocardiography shows progressive cardiac dilation over time in dnp38α, dnMKK3, and dnMKK6 transgenic mice (n = 4 each group). (c) Measurement of ANF and BNP mRNA levels in nontransgenic and transgenic hearts at 2 months of age averaged from four independent hearts. (d) Macroscopic histological analysis of H&E-stained hearts from dnp38α, dnMKK3, and dnMKK6 transgenic mice at 4 months of age (top panels) shows increased heart size in the transgenic mice. The middle panels show Masson’s trichrome staining at 4 months (×200), which reveals interstitial cell fibrosis in dnp38α and dnMKK3 transgenic hearts (blue). Histological sections were also stained with wheat germ agglutinin-TRITC conjugate (bottom panels) to permit quantitation (e) of myocyte cross-sectional areas (n = 200 cells per section) (*P < 0.05 versus nontransgenic mice).
Figure 3
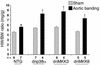
dnp38α, dnMKK3, and dnMKK6 transgenic mice have enhanced hypertrophy following aortic banding (pressure overload). Two-month-old nontransgenic littermates or dnp38α, dnMKK3, and dnMKK6 transgenic mice underwent 14 days of abdominal aortic constriction, after which the hearts were removed and weighed (normalized to body weight). The number of animals in each group is shown (*P < 0.05 versus nontransgenic sham controls; †P < 0.05 versus nontransgenic banded).
Figure 4
dnp38α, dnMKK3, and dnMKK6 transgenic mice have enhanced hypertrophy in response to PE, Ang II, and ISO infusion. Nontransgenic mice or the three transgenic lines were infused with PE, Ang II, or ISO for 14 days with osmotic minipumps, after which the hearts were collected and weighed. The number of mice used in each group is denoted in the graph (*P < 0.05 versus nontransgenic vehicle; †P < 0.05 versus dnp38α, dnMKK3, or dnMKK6 vehicle, respectively).
Figure 5
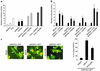
p38 signaling regulates NFAT transcriptional responses. (a) Transient transfections in neonatal cardiomyocytes using an NFAT-dependent luciferase reporter plasmid with vectors encoding either NFATc4, activated CnA, dnMKK3, or dnp38α and combinations thereof. The data demonstrate that p38 inhibition activates the NFAT reporter in cardiomyocytes (*P < 0.05 versus vector alone). (b) This p38 inhibitory effect was surveyed against all four NFAT factors in 10T1/2 cells (NFATc1-c4). The data demonstrate that dnp38α cotransfection activates NFATc1, NFATc2, and NFATc4 transcription nearly as well as activated calcineurin. Similar results were observed in two additional, independent experiments (*P < 0.05 versus NFAT alone). (c) Recombinant adenovirus encoding NFATc1-GFP was used to evaluate nuclear translocation in cultured cardiomyocytes. Control coinfection with adenovirus-encoding β-galactosidase (Adβgal) did not induce NFATc1-GFP nuclear translocation, distinct from the effect of CnA adenovirus (AdCnA; activated). The dnp38α-encoding adenovirus (Ad-dnp38α) induced significant NFATc1 nuclear translocation. These results were quantified in approximately 250 infected cardiomyocytes. Arrows, cells with cytoplasmic NFAT-GFP; arrowheads, cells with nuclear NFAT-GFP. Similar results were observed in three independent experiments (*P < 0.05 versus Adβgal).
Figure 6
Inhibition of p38 enhances the activity of an NFAT-dependent luciferase reporter transgene in vivo. (a) Schematic representation of the 9×NFAT-TATA-luciferase construct used to generate transgenic mice. (b) Relative luciferase (luc) levels from cardiac protein extracts from 3-week-, 2-month-, or 4-month-old nontransgenic mice (background), NFAT-luc transgenic mice, or double transgenic mice containing NFAT-luc and dnp38α. All data are shown relative to background (no transgene), which was set to a value of 1. (*P < 0.05 versus NFAT-luc alone).
Figure 7
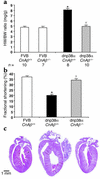
Calcineurin Aβ gene targeting blocks dnp38α-induced cardiac growth. (a) Measurement of heart-to-body weight ratios in 2-month-old dnp38α transgenic mice (FVB strain) crossed into the calcineurin Aβ wild-type or null background. (b) Echocardiography-measured fractional shortening shows a rescue in dnp38α transgene-induced functional decompensation by calcineurin Aβ gene targeting. Six mice were analyzed in each group. (c) Gross histological analysis also reveals a rescue in eccentric hypertrophic growth associated with p38 inhibition by calcineurin Aβ gene disruption (these hearts align with the indicated groups in b) (*P < 0.05 versus wild type; †P < 0.05 versus CnAβ+/+ mice with the dnp38α transgene).
Similar articles
- Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth.
Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD. Sanna B, et al. Mol Cell Biol. 2005 Feb;25(3):865-78. doi: 10.1128/MCB.25.3.865-878.2005. Mol Cell Biol. 2005. PMID: 15657416 Free PMC article. - c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling.
Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. Liang Q, et al. EMBO J. 2003 Oct 1;22(19):5079-89. doi: 10.1093/emboj/cdg474. EMBO J. 2003. PMID: 14517246 Free PMC article. - Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs.
Molkentin JD. Molkentin JD. Cardiovasc Res. 2004 Aug 15;63(3):467-75. doi: 10.1016/j.cardiores.2004.01.021. Cardiovasc Res. 2004. PMID: 15276472 Review. - Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy.
Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Wilkins BJ, et al. Circ Res. 2004 Jan 9;94(1):110-8. doi: 10.1161/01.RES.0000109415.17511.18. Epub 2003 Dec 1. Circ Res. 2004. PMID: 14656927 - Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes.
Fiedler B, Wollert KC. Fiedler B, et al. Cardiovasc Res. 2004 Aug 15;63(3):450-7. doi: 10.1016/j.cardiores.2004.04.002. Cardiovasc Res. 2004. PMID: 15276470 Review.
Cited by
- p38 MAPK Pathway in the Heart: New Insights in Health and Disease.
Romero-Becerra R, Santamans AM, Folgueira C, Sabio G. Romero-Becerra R, et al. Int J Mol Sci. 2020 Oct 8;21(19):7412. doi: 10.3390/ijms21197412. Int J Mol Sci. 2020. PMID: 33049962 Free PMC article. Review. - Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs).
Liu R, Molkentin JD. Liu R, et al. J Mol Cell Cardiol. 2016 Dec;101:44-49. doi: 10.1016/j.yjmcc.2016.08.018. Epub 2016 Aug 27. J Mol Cell Cardiol. 2016. PMID: 27575022 Free PMC article. Review. - Single Muscle Immobilization Decreases Single-Fibre Myosin Heavy Chain Polymorphism: Possible Involvement of p38 and JNK MAP Kinases.
Derbré F, Droguet M, Léon K, Troadec S, Pennec JP, Giroux-Metges MA, Rannou F. Derbré F, et al. PLoS One. 2016 Jul 6;11(7):e0158630. doi: 10.1371/journal.pone.0158630. eCollection 2016. PLoS One. 2016. PMID: 27383612 Free PMC article. - Protein Kinases as Drug Development Targets for Heart Disease Therapy.
Dhalla NS, Müller AL. Dhalla NS, et al. Pharmaceuticals (Basel). 2010 Jul 5;3(7):2111-2145. doi: 10.3390/ph3072111. Pharmaceuticals (Basel). 2010. PMID: 27713345 Free PMC article. Review. - The POU4F2/Brn-3b transcription factor is required for the hypertrophic response to angiotensin II in the heart.
Mele L, Maskell LJ, Stuckey DJ, Clark JE, Heads RJ, Budhram-Mahadeo VS. Mele L, et al. Cell Death Dis. 2019 Aug 14;10(8):621. doi: 10.1038/s41419-019-1848-y. Cell Death Dis. 2019. PMID: 31413277 Free PMC article.
References
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. - PubMed
- Ho KK, Levy D, Kannel WB, Pinsky JL. The epidemiology of heart failure: The Framingham Study. J. Am. Coll. Cardiol. 1993;22:6–13. - PubMed
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Engl. J. Med. 1990;322:1561–1566. - PubMed
- van Zwieten PA. The influence of antihypertensive drug treatment on the prevention and regression of left ventricular hypertrophy. Cardiovasc. Res. 2000;45:82–91. - PubMed
- Molkentin JD, Dorn GW. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous