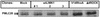A role for LIM kinase in cancer invasion - PubMed (original) (raw)
A role for LIM kinase in cancer invasion
Kiyoko Yoshioka et al. Proc Natl Acad Sci U S A. 2003.
Abstract
In this study, we show that LIM kinase 1 (LIMK1), a critical regulator of actin dynamics, plays a regulatory role in tumor cell invasion. We found that the level and activity of endogenous LIMK1 is increased in invasive breast and prostate cancer cell lines in comparison with less invasive cells. Overexpression of LIMK1 in MCF-7 and in MDA-MB-231 human breast cancer cell lines increased their motility, whereas the specific ROCK and Rho inhibitors Y-27632 and C3, respectively, attenuated this effect. In addition, inhibition of LIMK1 activity in the MDA-MB-231 cells by expression of dominant-negative LIMK1 resulted in decreased motility and formation of osteolytic bone lesions in an animal model of tumor invasion. This study shows an important role for LIMK1 signaling in invasion of cancer, demonstrating its potential as a therapeutic molecular target to decrease metastasis.
Figures
Fig. 1.
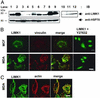
Immunoblot and immunofluorescence analyses of endogenous LIMK1 expression in cell lines. (A Upper) Endogenous LIMK1 expression in various cell lines (lanes 1–9), and after alkaline phosphatase treatment of cell lysates from transformed and invasive cancer cell lines (lanes 10–12). (Lower) HSP70 expression to indicate loading of proteins. Lane 1, mouse olfactory epithelial cells; lane 2, monkey COS-7 cells; lane 3, 293T cells; lane 4, mouse NIH 3T3 fibroblasts; lanes 5 and 10, Ras-transformed NIH 3T3 fibroblasts; lane 6, breast cancer MCF-7 cells; lane 7, prostate cancer LNCaP cells; lanes 8 and 12, invasive prostate cancer PC-3 cells; lanes 9 and 11, invasive breast cancer MDA-MB-231 cells. (B) LIMK1 and vinculin expression in MCF-7 (Upper) and MDA-MB-231 cells (Lower), and LIMK1 localization in both cell-types after a 30-min treatment with 10 μM Y-27632 (Right). (C) LIMK1 and F-actin expression in nontreated MCF-7 and MDA-MB-231 cells. (Bars = 20 μm.)
Fig. 2.
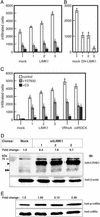
LIMK1 expression and activity in MDA-MB-231 transfectants. (A) Immunoblots of cell lysates prepared from mock, wtLIMK1, and DN-LIMK1 MDA-MB-231 transfectants probed with rat monoclonal anti-LIMK1 (Upper) or β-actin antibodies, as loading control (Lower). The fold-change in the level of LIMK1 protein compared with the mock transfectants is indicated above Upper. The positions of endogenous and overexpressed tagged-LIMK1 proteins are indicated by double and single arrowheads, respectively. (B) Immunoblots of cell lysates prepared from mock, wtLIMK1, and DN-LIMK1 MDA-MB-231 transfectants probed with anti-phospho-cofilin (Upper) and pancofilin (Lower) antibodies. The fold-change in phosphorylated cofilin level is indicated above Upper. The data are representative of three independent experiments.
Fig. 3.
The invasiveness of LIMK1 MDA-MB-231 and MCF-7 transfectants. Cells from each cell line (5 × 105 cells) were added onto the upper chamber of Matrigel-coated PET membrane. (A) For MDA-MB-231 mock transfectants or overexpressing wtLIMK1 (LIMK1), cells were left untreated or were treated with Y-27632 (10 μM) and added directly to the assay system, or pretreated for 24 h with 10 μg/ml C3. The number of migrating cells was determined after 6h.(B) Migration of MDA-MB-231 DN-LIMK1 or mock transfectants. (C) MCF-7 transfectants expressing wtLIMK1 (LIMK1) or V14RhoA and Δ4ROCK were analyzed after 20 h. The numbers at the bottom of each panel indicate the clone's number. (D) Immunoblots of cell lysates prepared from mock and wtLIMK1 MCF-7 transfectants probed with rat monoclonal anti-LIMK1 (Upper) and β-actin (Lower) antibodies. The fold-change in the level of LIMK1 protein compared with the mock transfectants is indicated above Upper. The positions of endogenous and overexpressed tagged-LIMK1 proteins are indicated by double and single arrowheads, respectively. (E) Immunoblots of cell lysates prepared from mock and wtLIMK1 MCF-7 transfectants probed with anti-phospho-cofilin (Upper) and pan-cofilin (Lower) antibodies. The fold-change in phosphorylated cofilin compared with the mock transfectants is indicated above Lower. All data are expressed as the number of migrated cells and are the average of three independent experiments. Error bars indicate SD. *, P < 0.01 to mock transfectants; #, P < 0.01 to Y (-) each transfectant.
Fig. 4.
Phosphorylated MLC-20 levels in MCF-7 transfectants. Immunoblots of cell lysates prepared from mock, wtLIMK1, V14RhoA, and Δ4ROCK MCF-7 transfectants probed with anti-phospho-MLC-20 antibodies. The position of phosphorylated MLC-20 (PMLC20) is indicated by arrows.
Fig. 5.
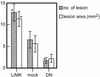
Osteolytic lesions caused by LIMK1-MDA-MB-231 cells in nude mice. The number and area of osteolytic lesions in nude mice bearing wtLIMK1-MDA-MB-231 (clone 3; 11 mice), DN-LIMK1 (clone 3; 11 mice), and mock (14 mice) transfected cells. The number and area of osteolytic lesions were scored 3 wk after cell inoculation on the radiographs by using quantitative image analysis.
Similar articles
- Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone.
Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Liu S, et al. Cancer Res. 2009 Nov 15;69(22):8742-51. doi: 10.1158/0008-5472.CAN-09-1541. Epub 2009 Nov 3. Cancer Res. 2009. PMID: 19887617 - Expression of LIM kinase 1 is associated with reversible G1/S phase arrest, chromosomal instability and prostate cancer.
Davila M, Jhala D, Ghosh D, Grizzle WE, Chakrabarti R. Davila M, et al. Mol Cancer. 2007 Jun 8;6:40. doi: 10.1186/1476-4598-6-40. Mol Cancer. 2007. PMID: 17559677 Free PMC article. - LIM kinase 1 increases tumor metastasis of human breast cancer cells via regulation of the urokinase-type plasminogen activator system.
Bagheri-Yarmand R, Mazumdar A, Sahin AA, Kumar R. Bagheri-Yarmand R, et al. Int J Cancer. 2006 Jun 1;118(11):2703-10. doi: 10.1002/ijc.21650. Int J Cancer. 2006. PMID: 16381000 - RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes.
Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. Armstrong AP, et al. Prostate. 2008 Jan 1;68(1):92-104. doi: 10.1002/pros.20678. Prostate. 2008. PMID: 18008334 - LIM-kinase1.
Stanyon CA, Bernard O. Stanyon CA, et al. Int J Biochem Cell Biol. 1999 Mar-Apr;31(3-4):389-94. doi: 10.1016/s1357-2725(98)00116-2. Int J Biochem Cell Biol. 1999. PMID: 10224665 Review.
Cited by
- Laminin-5 beta3A expression in LNCaP human prostate carcinoma cells increases cell migration and tumorigenicity.
Calaluce R, Bearss DJ, Barrera J, Zhao Y, Han H, Beck SK, McDaniel K, Nagle RB. Calaluce R, et al. Neoplasia. 2004 Sep-Oct;6(5):468-79. doi: 10.1593/neo.03499. Neoplasia. 2004. PMID: 15548355 Free PMC article. - LIMK1 Interacts with STK25 to Regulate EMT and Promote the Proliferation and Metastasis of Colorectal Cancer.
Sun X, Li S, Lin H. Sun X, et al. J Oncol. 2022 Feb 28;2022:3963883. doi: 10.1155/2022/3963883. eCollection 2022. J Oncol. 2022. PMID: 35265128 Free PMC article. - Sodium butyrate induces senescence and inhibits the invasiveness of glioblastoma cells.
Nakagawa H, Sasagawa S, Itoh K. Nakagawa H, et al. Oncol Lett. 2018 Feb;15(2):1495-1502. doi: 10.3892/ol.2017.7518. Epub 2017 Dec 5. Oncol Lett. 2018. PMID: 29434841 Free PMC article. - Integrative analysis of mutational and transcriptional profiles reveals driver mutations of metastatic breast cancers.
Lee JH, Zhao XM, Yoon I, Lee JY, Kwon NH, Wang YY, Lee KM, Lee MJ, Kim J, Moon HG, In Y, Hao JK, Park KM, Noh DY, Han W, Kim S. Lee JH, et al. Cell Discov. 2016 Aug 30;2:16025. doi: 10.1038/celldisc.2016.25. eCollection 2016. Cell Discov. 2016. PMID: 27625789 Free PMC article. - Novel anti-HIV therapeutics targeting chemokine receptors and actin regulatory pathways.
Spear M, Guo J, Wu Y. Spear M, et al. Immunol Rev. 2013 Nov;256(1):300-12. doi: 10.1111/imr.12106. Immunol Rev. 2013. PMID: 24117829 Free PMC article. Review.
References
- Chambers, A. F., Groom, A. C. & MacDonald, I. C. (2002) Nat. Rev. Cancer 2, 563-572. - PubMed
- Vivanco, I. & Sawyers, C. L. (2002) Nat. Rev. Cancer 2, 489-501. - PubMed
- Roymans, D. & Slegers, H. (2001) Eur. J. Biochem. 268, 487-498. - PubMed
- Seasholtz, T. M., Majumdar, M. & Brown, J. H. (1999) Mol. Pharmacol. 55, 949-956. - PubMed
- Muller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., McClanahan, T., Murphy, E., Yuan, W., Wagner, S. N., et al. (2001) Nature 410, 50-56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous