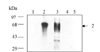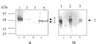Uptake of proteins and degradation of human serum albumin by Plasmodium falciparum-infected human erythrocytes - PubMed (original) (raw)
Uptake of proteins and degradation of human serum albumin by Plasmodium falciparum-infected human erythrocytes
Ahmed El Tahir et al. Malar J. 2003.
Abstract
Background: Intraerythrocytic malaria parasites actively import obligate nutrients from serum and export proteins and lipids to erythrocyte cytoplasm and membrane. The import of macromolecules in the malaria parasite has been the subject of many debates. To understand the import of macromolecules by the parasite, we studied the uptake of proteins by Plasmodium falciparum infected human erythrocyte.
Methods: Proteins were biotin labelled individually, purified on a gel filtration column and added to uninfected and infected asynchronized culture. The uptake of these proteins by malaria parasites was determined by western blot analysis of parasite pellet and their different fractions using streptavidin-horseradish conjugate. To further confirm this import, we studied the uptake of 125I-labelled proteins by western blot analysis as well as used direct immunofluorescence method.
Results: Here we show that biotin labelled and radio-iodinated polypeptides of molecular sizes in the range of 45 to 206 kDa, when added in the culture medium, get direct access to the parasite membrane through a membrane network by by-passing the erythrocyte cytosol. The import of these polypeptides is ATP-dependent as sodium azide treatment blocks this uptake. We also show that malaria parasites have the ability to take up and degrade biotin labelled human serum albumin, which has been shown to be essential for the parasite growth.
Conclusions: These results can be used, as a basis to explore the role of human serum albumin in the intraerythrocytic development of parasites, and this in turn can be an important adjunct to the development of novel antimalarial drugs.
Figures
Figure 1
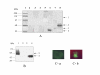
Uptake of high molecular weight proteins by infected human erythrocytes (A) Western blot analysis of biotin labelled proteins in different fractions of saponin lysed parasitized RBC and uninfected RBCs using streptavidin horseradish peroxidate conjugate. Protein markers (lane 1), saponin treatment lysate of uninfected RBCs incubated with labelled PfHRP-2 (lane 2), intact parasite pellet of infected RBCs incubated with unlabelled recombinant PfHRP-2 (lane 3), supernatant of infected RBCs treated with labelled PfHRP-2 (lane 4), parasite pellet of infected RBC incubated with labelled β galactosidase (lane 5), egg albumin (lane 6), β amylase (tetramer) (lane 7) and recombinant PfHRP-2 (lane 8). (B) SDS-PAGE analysis of radio-iodinated proteins imported by the parasite from the culture. Saponin lysed intact parasite pellet incubated with recombinant 125I PfHRP-2 (lane 1) and 125I egg albumin (lane 4). Supernatant of saponin lysed infected RBC incubated with 125I PfHRP-2 (lane 2) and 125I egg albumin (lane 3). (C) Direct immunofluorescence analysis to show the uptake of HAS by infected human erythrocytes. (A) uninfected RBCs incubated with biotin labelled protein and (B) infected RBCs incubated with biotin labelled protein.
Figure 2
Western blot analysis to show location of imported biotin labelled PfHRP-2 in different compartments of parasite. Supernatant after saponin lysis of infected RBC (lane 1), purified intact parasite pellet (lane 2), Triton X-100 insoluble parasite membrane fraction (lane 3), parasite cytoplasm (lane 4).
Figure 3
(A), Western blot analysis to show the effect of sodium azide on the import of biotinylated HSA and PfHRP-2 to the infected human red blood cells. (A), Uptake of HSA by untreated parasite culture (lane 1) uptake of HSA after sodium azide treatment (lane 2). Uptake of PfHRP-2 by sodium azide treated parasite culture (lane 3), uptake of PfHRP-2 by untreated parasite culture (lane 4). (B), Western blot analysis to show the effects of BFA and monensin to the uptake of biotinylated PfHRP-2. Parasite pellets of untreated culture (lane 1), BFA treated culture (lane 2) and monesnin treated culture (lane 3).
Figure 4
Western blot analysis of saponin lysed parasite pellet to show the uptake of biotinylated egg albumin after 30 min (lane 1) and after 12 h (lane 2), PfHRP-2 after 30 min (lane 3) and 12 h (lane 4) and HSA after 30 min (lane 5) and 12 h (lane 6) of incubation.
Figure 5
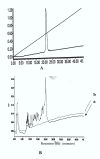
Incubation of HSA with trophozoite lysate generates discrete fragments. Reverse phase chromatography profile of HSA[A] trophozoite lysate alone [B (b)] and HSA plus trophozoite lysate incubated overnight [B (a)].
Similar articles
- A homologue of Sar1p localises to a novel trafficking pathway in malaria-infected erythrocytes.
Albano FR, Berman A, La Greca N, Hibbs AR, Wickham M, Foley M, Tilley L. Albano FR, et al. Eur J Cell Biol. 1999 Jul;78(7):453-62. doi: 10.1016/S0171-9335(99)80072-7. Eur J Cell Biol. 1999. PMID: 10472798 - Methionine transport in the malaria parasite Plasmodium falciparum.
Cobbold SA, Martin RE, Kirk K. Cobbold SA, et al. Int J Parasitol. 2011 Jan;41(1):125-35. doi: 10.1016/j.ijpara.2010.09.001. Epub 2010 Sep 17. Int J Parasitol. 2011. PMID: 20851123 - Lipid transport in Plasmodium.
Haldar K. Haldar K. Infect Agents Dis. 1992 Oct;1(5):254-62. Infect Agents Dis. 1992. PMID: 1344664 Review. - Pexel/VTS: a protein-export motif in erythrocytes infected with malaria parasites.
Horrocks P, Muhia D. Horrocks P, et al. Trends Parasitol. 2005 Sep;21(9):396-9. doi: 10.1016/j.pt.2005.07.004. Trends Parasitol. 2005. PMID: 16046186 Review.
Cited by
- Plug for the parasitophorous duct: a solution of two conundra.
Wilairat P, Auparakkitanon S. Wilairat P, et al. Malar J. 2020 Oct 16;19(1):370. doi: 10.1186/s12936-020-03445-9. Malar J. 2020. PMID: 33066767 Free PMC article. - Cultivation of Asexual Intraerythrocytic Stages of Plasmodium falciparum.
Basco LK. Basco LK. Pathogens. 2023 Jul 1;12(7):900. doi: 10.3390/pathogens12070900. Pathogens. 2023. PMID: 37513747 Free PMC article. Review. - Molecular Camouflage of Plasmodium falciparum Merozoites by Binding of Host Vitronectin to P47 Fragment of SERA5.
Tougan T, Edula JR, Takashima E, Morita M, Shinohara M, Shinohara A, Tsuboi T, Horii T. Tougan T, et al. Sci Rep. 2018 Mar 22;8(1):5052. doi: 10.1038/s41598-018-23194-9. Sci Rep. 2018. PMID: 29567995 Free PMC article. - Sphingosine 1-Phosphate in Malaria Pathogenesis and Its Implication in Therapeutic Opportunities.
Dhangadamajhi G, Singh S. Dhangadamajhi G, et al. Front Cell Infect Microbiol. 2020 Aug 14;10:353. doi: 10.3389/fcimb.2020.00353. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32923406 Free PMC article. Review. - Recent Updates on Interaction Studies and Drug Delivery of Antimalarials with Serum Albumin Proteins.
Azeem K, Irfan I, Rashid Q, Singh S, Patel R, Abid M. Azeem K, et al. Curr Med Chem. 2024;31(25):3925-3953. doi: 10.2174/0929867330666230509121931. Curr Med Chem. 2024. PMID: 37218197 Review.
References
- Ginsburg H, Kirk K. Membrane transport in the malaria – infected erythrocytes. In "Malaria". ASM Press, Washington DC. 1998. pp. 219–232.
- Sherman IW. Membrane structure and function of malaria parasites and the infected erythrocyte. Parasitology. 1985;91:606–645. - PubMed
- Sherman IW. Mechanism of molecular trafficking in malaria. Parasitology. 1988;96:857–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources