The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells - PubMed (original) (raw)
The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells
Sreekanth H Chalasani et al. J Neurosci. 2003.
Abstract
The chemokine receptor CXCR4 is expressed in the embryonic and mature CNS, yet its normal physiological function in neurons remains obscure. Here, we show that its cognate chemokine, stromal cell-derived factor-1 (SDF-1), promotes the survival of cultured embryonic retinal ganglion cell neurons even in the absence of other neurotrophic factors. This survival effect is mediated primarily through a cAMP-dependent pathway that acts through protein kinase A and MAP kinase. Addition of SDF-1 to a human neuronal cell line induces phosphorylation of p44/p42 MAP kinase and GSK3beta. Mouse embryos lacking the CXCR4 receptor have a reduced number of retinal ganglion cells. The ligand of CXCR4, SDF-1, may therefore provide generalized trophic support to neurons during their development and maturation.
Figures
Figure 1.
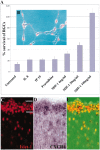
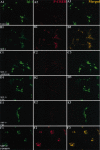
SDF-1 promotes the survival of cultured embryonic chick RGCs, and its receptor CXCR4 is expressed in the embryonic retina. A, SDF-1 promotes the survival of RGCs in a dose-dependent manner. Shown are the percentages of surviving RGCs at 72 compared with 24 hr in the presence of 100 ng/ml selected chemokines or of 4 (p = 0.028), 20 (p = 0.008), or 100 ng/ml (p = 0.001) SDF-1. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, Identification of RGCs in culture with anti-islet-1. C, Visualization of RGC neurons with an antibody to islet-1 in a cross section of an E6 chick embryo. The hash marks represent the ganglion cell layer. D, Visualization of CXCR4-expressing cells in the RGC layer of this same section by in situ hybridization. E, Merged image showing CXCR4 in all islet-1-positive cells.
Figure 3.
SDF promotes the survival of cultured RGCs without promoting the translocation of TrkB to the cell surface. A, RGC survival is enhanced by 10 μ
m
forskolin (p = 0.002) to the same degree as by 100 ng/ml SDF-1 (p = 0.001). The average percentages of surviving RGCs at 72 hr compared with 24 hr are shown for three independent experiments. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, SDF-1-promoted survival is not affected by the src family inhibitor PP1, whereas BDNF survival is completely blocked. C–E, Forskolin but not SDF-1 induces the translocation of TrkB to the surface of RGCs. After 24 hr, cultures were left untreated (C1, C2) or treated for 30 min with 10 μ
m
forskolin (D1,D2) or 100 ng/ml SDF-1 (E1,E2). All cultures were then stained live with anti-rabbit TrkB antibody. RGCs are visualized in green with anti-islet-1 (C1–E1), and surface TrkB is visualized in the same fields in red (C2–E2) (20×). Although both forskolin and SDF-1 promote the survival of RGCs, forskolin induces the translocation of TrkB to the surface of retinal cells, whereas SDF-1 does not.
Figure 2.
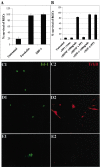
SDF-1 reduces the percentage of TUNEL-positive RGCs. Dissociated E6 chick neural retinas were cultured for 48 hr, fixed, and processed for TUNEL staining (red) and islet-1 expression (green). A representative field is shown in phase-contrast optics (A) and in fluorescence (B). Some islet-1-expressing nuclei are also TUNEL-positive (arrows). C, The percentages of islet-1-positive cells with TUNEL staining were determined in the presence or the absence of 100 ng/ml SDF-1 (p = 0.002). The average percentages of TUNEL-positive RGCs are shown from four independent experiments. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. Approximately threefold more TUNEL-positive RGCs were detected in the absence than in the presence of SDF-1.
Figure 4.
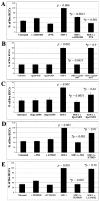
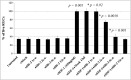
Signaling pathways required for SDF-1-mediated RGC survival. The average number of RGCs per field 48 hr after plating is shown for each condition in four independent experiments. Each inhibitor was tested in the presence or absence of 100 ng/ml SDF-1. A, AMD3100 (20 μ
m
), an antagonist of SDF-1 binding to CXCR4, does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. The Gi inhibitor PTX (100 ng/ml) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. B, HxB-gp120 (100 ng/ml), anantagonist of SDF-1 binding to CXCR4, does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. JRFL-gp120 (100 ng/ml) does not antagonize SDF-1 binding and does not block its survival effect C, The cAMP antagonist Rp-cAMPS (20 μ
m
) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. The cGMP antagonist Rp-cGMPs (20 μ
m
) has a lesser effect on SDF-1 activity than the cAMP antagonist. D, The PKA inhibitor PKI (200 n
m
) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1, whereas a 1 μ
m
concentration of the PKG inhibitor KT5823 is less effective in blocking the effect of SDF-1. E, The survival effect of SDF-1 is also blocked by a 20 μ
m
concentration of the MAP kinase inhibitor PD98059. In contrast, a 20 μ
m
concentration of the PI-3 kinase inhibitor LY294002 does not reduce the effect of SDF-1. p values were calculated by comparing each population with the untreated one, and those with an asterisk were obtained comparing each population with the SDF-1-treated one using a two-tailed test with different variances.
Figure 5.
SDF-1 induces phosphorylation and translocation of CREB into the nucleus. E6 retinal neurons were cultured in defined minimal medium for 24 hr (A1–A3) and then exposed to 100 ng/ml SDF-1 (B1–B3), SDF-1 plus a 20 μ
m
concentration of the CXCR4 antagonist AMD3100 (C1–C3), SDF-1 plus 100 ng/ml PTX (D1–D3), SDF-1 plus a 200 n
m
concentration of the PKA inhibitor PKI (E1–E3), or SDF-1 plus a 20 μ
m
concentration of MAP kinase inhibitor PD98059 (F1–F3). After 30 min, the cultures were fixed and stained for islet-1 (A1–F1) (green) and phosphorylated CREB (A2–F2) (red). Merged images are shown in A3–F3. SDF-1 induces translocation of phosphorylated CREB into the nuclei of retinal neurons that is blocked by all three pharmacological agents.
Figure 6.
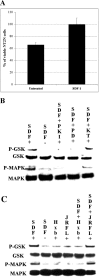
SDF-1 enhances survival and induces phosphorylation of MAP kinase and GSK3β in NT2N cells. A, SDF-1 100 ng/ml (p = 0.028) increases the number of NT2N cells after 24 hr in serum-free medium. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, SDF-1 enhances phosphorylation of GSK3β and MAP kinase 44/42. The PKA inhibitor PKI at 20 μ
m
blocks SDF-1-induced phosphorylation of GSK3β and MAP kinase. The MAP kinase inhibitor PD98059 at 20 μ
m
also blocks the phosphorylation of both GSK3β and MAP kinase. The PKG antagonist KT5823 at 1 μ
m
does not block SDF-1-induced phosphorylation of GSK3β and MAP kinase. C, Neither 100 ng/ml HxB-gp120 nor JRFL-gp120 affects GSK3β or MAP kinase phosphorylation on their own. HxB-gp120 but not JRFL-gp120 blocks SDF-1-induced phosphorylation of GSK3β and MAP kinase.
Figure 7.
SDF-1-induced survival is blocked by slit-2. Increasing concentrations of slit-2 (≥6 collapsing units) can block SDF-1-induced survival. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances.
Figure 8.
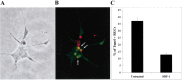
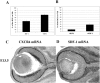
SDF-1 enhances the survival of embryonic mouse RGCs. After 24 (A; p = 0.001) or 72 (B; p = 0.005) hr in culture, an increased percentage of RGCs survive in the presence of 100 ng/ml SDF-1. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. C, CXCR4 mRNA is expressed in the RGC cell layer of E13.5 mouse retina. D, At the same embryonic stage, SDF-1 mRNA is expressed in connective tissues surrounding the eye.
Figure 9.
Mice lacking CXCR4 have fewer RGCs. A, Confocal reconstruction of a representative 2 μm optical section from an E13.5 wild-type eye. B, Table comparing the number of islet-1-positive RGCs (see Materials and Methods) in wild-type and CXCR4 mutant eyes. The number of RGCs in mutant embryos is estimated to be ∼65% of those in wild-type littermates.
Similar articles
- A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding.
Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. Chalasani SH, et al. J Neurosci. 2003 Feb 15;23(4):1360-71. doi: 10.1523/JNEUROSCI.23-04-01360.2003. J Neurosci. 2003. PMID: 12598624 Free PMC article. - Stromal cell-derived factor-1 (SDF-1) signalling regulates human placental trophoblast cell survival.
Jaleel MA, Tsai AC, Sarkar S, Freedman PV, Rubin LP. Jaleel MA, et al. Mol Hum Reprod. 2004 Dec;10(12):901-9. doi: 10.1093/molehr/gah118. Epub 2004 Oct 8. Mol Hum Reprod. 2004. PMID: 15475370 - Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes.
Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Lazarini F, et al. Eur J Neurosci. 2000 Jan;12(1):117-25. doi: 10.1046/j.1460-9568.2000.00894.x. Eur J Neurosci. 2000. PMID: 10651866 - Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation.
Tang Z, Arjunan P, Lee C, Li Y, Kumar A, Hou X, Wang B, Wardega P, Zhang F, Dong L, Zhang Y, Zhang SZ, Ding H, Fariss RN, Becker KG, Lennartsson J, Nagai N, Cao Y, Li X. Tang Z, et al. J Exp Med. 2010 Apr 12;207(4):867-80. doi: 10.1084/jem.20091704. Epub 2010 Mar 15. J Exp Med. 2010. PMID: 20231377 Free PMC article. - Chemokine signaling guides axons within the retina in zebrafish.
Li Q, Shirabe K, Thisse C, Thisse B, Okamoto H, Masai I, Kuwada JY. Li Q, et al. J Neurosci. 2005 Feb 16;25(7):1711-7. doi: 10.1523/JNEUROSCI.4393-04.2005. J Neurosci. 2005. PMID: 15716407 Free PMC article.
Cited by
- Macrophage migration inhibitory factor acts as a neurotrophin in the developing inner ear.
Bank LM, Bianchi LM, Ebisu F, Lerman-Sinkoff D, Smiley EC, Shen YC, Ramamurthy P, Thompson DL, Roth TM, Beck CR, Flynn M, Teller RS, Feng L, Llewellyn GN, Holmes B, Sharples C, Coutinho-Budd J, Linn SA, Chervenak AP, Dolan DF, Benson J, Kanicki A, Martin CA, Altschuler R, Koch AE, Jewett EM, Germiller JA, Barald KF. Bank LM, et al. Development. 2012 Dec;139(24):4666-74. doi: 10.1242/dev.066647. Development. 2012. PMID: 23172918 Free PMC article. - The longevity-associated variant of BPIFB4 improves a CXCR4-mediated striatum-microglia crosstalk preventing disease progression in a mouse model of Huntington's disease.
Di Pardo A, Ciaglia E, Cattaneo M, Maciag A, Montella F, Lopardo V, Ferrario A, Villa F, Madonna M, Amico E, Carrizzo A, Damato A, Pepe G, Marracino F, Auricchio A, Vecchione C, Maglione V, Puca AA. Di Pardo A, et al. Cell Death Dis. 2020 Jul 18;11(7):546. doi: 10.1038/s41419-020-02754-w. Cell Death Dis. 2020. PMID: 32683420 Free PMC article. - Treatment with AMD3100 attenuates the microglial response and improves outcome after experimental stroke.
Walter HL, van der Maten G, Antunes AR, Wieloch T, Ruscher K. Walter HL, et al. J Neuroinflammation. 2015 Feb 7;12:24. doi: 10.1186/s12974-014-0232-1. J Neuroinflammation. 2015. PMID: 25881123 Free PMC article. - Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo.
Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, Granato M, Chien CB, Raper JA. Chalasani SH, et al. J Neurosci. 2007 Jan 31;27(5):973-80. doi: 10.1523/JNEUROSCI.4132-06.2007. J Neurosci. 2007. PMID: 17267551 Free PMC article. - Stromal derived factor-1 exerts differential regulation on distinct cortical cell populations in vitro.
Pritchett J, Wright C, Zeef L, Nadarajah B. Pritchett J, et al. BMC Dev Biol. 2007 Apr 10;7:31. doi: 10.1186/1471-213X-7-31. BMC Dev Biol. 2007. PMID: 17425785 Free PMC article.
References
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA ( 1996) CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272: 1955–1958. - PubMed
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ ( 2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249–4260. - PubMed
- Barde Y-A ( 1989) Trophic factors and neuronal survival. Neuron 2: 1525–1534. - PubMed
- Berger EA, Murphy PM, Farber JM ( 1999) Chemokine receptors as HIV-1 co-receptors: roles in viral entry, tropism and disease. Annu Rev Immunol 17: 657–700. - PubMed
- Bernstein M, Lichtman JW ( 1999) Axonal atrophy: the retraction reaction. Curr Opin Neurobiol 9: 364–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous