Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707 - PubMed (original) (raw)
Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707
Judith Haendeler et al. Mol Cell Biol. 2003 Jul.
Abstract
The regulation of telomerase reverse transcriptase (TERT) plays an important role in the proliferative capacity and survival of cells. Here, we report that exogenously as well as endogenously induced oxidative stress leads to translocation of endogenous as well as overexpressed human TERT from the nucleus into the cytosol. TERT is transported through the nuclear pores in a leptomycin-sensitive and Ran GTPase-dependent process. H(2)O(2)-induced nuclear export of TERT is preceded by TERT tyrosine phosphorylation at position 707 and prevented by the Src kinase family inhibitor PP1. Oxidative stress-induced nuclear export of TERT depends on association with the Ran GTPase. In contrast, mutation of tyrosine 707 inhibits phosphorylation induced by oxidative stress and prevents association with Ran and nuclear export of TERT. Moreover, inhibition of tyrosine phosphorylation at 707 increases the antiapoptotic capacity of TERT. Taken together, depletion of nuclear TERT by tyrosine phosphorylation-dependent nuclear export of TERT is a novel mechanism for regulation of TERT localization, which reduces the antiapoptotic activity of TERT.
Figures
FIG. 1.
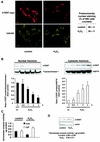
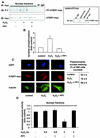
Exogenous oxidative stress induces translocation of hTERT. (A) A representative immunostaining using confocal microscopy is shown for 293 cells overexpressing myc-tagged hTERTwt with or without treatment with 500 μM H2O2 for 3 h. (Upper panels) hTERT-myc staining (red). (Lower panels) Tubulin staining to visualize the cell structure (green). Results from five different transfected dishes are shown. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (B) 293 cells were incubated with H2O2 (500 μM) for the indicated times, and nuclear and cytosolic fractions were separated. Immunoblotting was performed with an antibody against hTERT, and equal loading was confirmed either with topoisomerase 1 (nuclear fractions) or HSP90 (cytosolic fractions) (n = 4). Blots were scanned and semiquantitatively analyzed (lower panels). co, control. (C) Telomerase enzyme activity was measured in nuclear (nuc) and cytosolic (cyt) fractions of cells treated with 500 μM H2O2 for 3 h (n = 3 to 4). (D) 293 cells were incubated with H2O2 for 3 h, and hTERT protein levels were measured in whole-cell lysates (n = 3). A representative Western blot is shown. Equal loading was confirmed with actin. Enzymatic activity of the samples is shown in the lower part of panel D.
FIG.2.
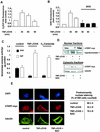
Endogenous oxidative stress induces nuclear export of hTERT. (A) 293 cells were incubated for the indicated times with 100 ng of TNF-α per ml and 10 μg of CHX per ml (TNF-α/CHX). Production of endogenous ROS was measured with H2DCF-DA by FACS analysis (n = 3). (B) After preincubation with PEG-SOD for 12 h, 293 cells were incubated for the indicated times with TNF-α/CHX (n = 3). Production of endogenous ROS was measured with DHE by FACS analysis. (C) Telomerase enzyme activity was measured in nuclear (nuc) and cytosolic (cyt) fractions of cells treated with 10 μM C2-ceramide for 6 h or TNF-α/CHX for 1 h (n = 3 to 4). (D) After preincubation with 10 mM NAC for 1 h, 293 cells overexpressing myc-tagged, full-length hTERT (hTERT-myc) were incubated with TNF-α/CHX for 1 h, and nuclear and cytosolic fractions were separated. Immunoblotting was performed with an antibody against myc, and equal loading was confirmed either with topoisomerase 1 or tubulin. (n = 3). (E) A representative immunostaining is shown for 293 cells overexpressing myc-tagged hTERTwt either untreated or treated with TNF-α/CHX for 1 h and 10 mM NAC as indicated. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side.
FIG. 3.
Nuclear export of hTERT occurs through the nuclear pores. (A) A representative immunostaining is shown for 293 cells overexpressing full-length, myc-tagged hTERT. Cells were incubated with 500 μM H2O2 for 3 h or were preincubated with 10 ng of leptomycin B (LMB) per ml for 30 min followed by 500 μM H2O2 for 3 h. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (B) 293 cells overexpressing full-length, myc-tagged hTERT (hTERT-myc) were preincubated with 10 ng of LMB per ml for 30 min and incubated with H2O2 for 3 h. Immunoblotting of the nuclear and cytosolic fractions was performed with an antibody against myc (upper panel), topoisomerase 1 as a loading control for nuclear fractions (middle panel), and HSP70 for cytosolic fractions (lower panels) (n = 4). The bar graphs below show the densitometric analysis (n = 4). (C) Telomerase enzyme activity was measured in nuclear and cytosolic fractions of cells in the presence or absence of 500 μM H2O2 or 500 μM H2O2 and 10 ng of LMB per ml. A representative telomeric repeat amplification product assay is shown. (D) Telomerase enzyme activity was measured in nuclear and cytosolic fractions of cells in the presence or absence of 500 μM H2O2 or 500 μM H2O2 plus 10 ng of LMB per ml (n = 3).
FIG. 4.
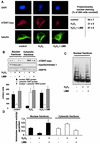
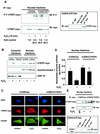
Nuclear export of hTERT is dependent on the GTPase Ran. (A) Lysates from 293 cells overexpressing myc-tagged, full-length hTERT were immunoprecipitated (IP) with an anti-myc antibody (left panels), an anti-Ran antibody, or an anti-mouse immunoglobulin G (IgG) antibody as indicated. Immunoblot (IB) analysis was performed against anti-Ran (lower panels) or anti-myc (upper panels) antibody. The right panels show the quality of the immunoprecipitations using an anti-myc or anti-Ran antibody. The input lane represents 1/10 of the cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) Lysates from 293 cells overexpressing myc-tagged hTERTwt were immunoprecipitated with an antimouse IgG or an anti-myc antibody as indicated, and immunoblot analysis was performed with an anti-CRM-1 antibody (upper panel). Membranes were stripped and reprobed with an anti-myc antibody (lower panel). The input lane represents 1/10 of the cell lysate used for immunoprecipitation. (C) A representative immunostaining is shown from cells overexpressing myc-tagged LacZ or myc-tagged Ran Q69L (dominant negative) incubated with H2O2 for 3 h. Cells were stained with TO-PRO-3 iodide to visualize the nuclei (blue, upper panel) and anti-TERT antibody followed by a goat anti-rabbit FITC-conjugated antibody (green, second upper panel) and anti-myc antibody (red, second lower panel). Cells were visualized by confocal microscopy. (D) A representative immunostaining is shown from cells overexpressing myc-tagged Ran G19V (active). Cells were stained with DAPI to visualize the nuclei (blue, left panel) and anti-TERT antibody followed by a biotin-labeled secondary antibody and streptavidin-FITC (green, second left panel) and anti-myc antibody (red, second right panel). (E) Nuclear and cytosolic lysates of cells overexpressing Ran Q69L or Ran G19V were dissolved by SDS-PAGE. Immunoblotting was performed with an antibody against hTERT (upper panels), and equal loading was confirmed either with topoisomerase 1 (nuclear fractions) or tubulin (cytosolic fractions) (n = 4) (middle panels). Densitometric analysis of four independent experiments is shown (lower panels). (F) Telomerase enzyme activity was measured in nuclear lysates of cells overexpressing LacZ, Ran Q69L, Ran G19V, or Ran T24N (n = 4) incubated in the presence or absence of H2O2 for 3 h.
FIG. 5.
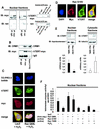
Src kinase family-dependent tyrosine phosphorylation plays a role in nuclear export of hTERT. (A) 293 cells overexpressing myc-tagged hTERTwt were incubated with 500 μM H2O2 in the presence or absence of 10 μM specific Src kinase family inhibitor PP1 for 15 min. Lysates of nuclear fractions were immunoprecipitated (IP) with an anti-myc antibody, and immunoblot (IB) analysis was performed with an antiphosphotyrosine antibody (upper panel). Immunoprecipitations were controlled by immunoblotting with anti-myc antibody (lower panel). A representative immunoblot is shown. The right panel shows the quality of the immunoprecipitation with an anti-myc antibody. The input lane represents 1/10 of cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) Densitometric analysis of three independent experiments is shown. (C) 293 cells overexpressing myc-tagged hTERTwt were incubated with 500 μM H2O2 in the presence or absence of 10 μM specific Src kinase family inhibitor PP1 for 2 h. A representative immunostaining is shown. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (D) Telomerase enzyme activity was measured in nuclear fractions of cells treated with 500 μM H2O2 or 500 μM H2O2 and 10 μM PP1 for the indicated times. Densitometric analysis is shown (n = 3).
FIG. 6.
Phosphorylation at tyrosine 707 signals nuclear export of hTERT. (A) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with 500 μM H2O2 for 15 min. Lysates of nuclear fractions were immunoprecipitated (IP) with an anti-myc antibody, and immunoblot (IB) analysis was performed with an antiphosphotyrosine antibody (PY [upper panel]). Immunoprecipitations were controlled by immunoblotting with anti-myc antibody (lower panel). A representative immunoblot is shown. The right panel shows the quality of the immunoprecipitation using an anti-myc antibody. The input lane represents 1/10 of cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h. Immunoblotting of the nuclear and cytosolic fractions was performed with an antibody against myc (upper panel), topoisomerase 1 (middle panel), and HSP70 (lower panel). Representative immunoblots are shown (n = 5). (C) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with 500 μM H2O2 for 2 h. A representative immunostaining is shown. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). (D) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h, and telomerase enzyme activity in the nuclear fractions was measured. Densitometric analysis is shown (n = 3). *, P < 0.05 versus hTERTwt plus H2O2. (E) Lysates from 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h. Cell lysates were immunoprecipitated with an anti-myc antibody, and immunoblot analysis was performed with an anti-Ran antibody (middle panel) and an anti-myc antibody (upper panel). The lower panel shows the quality of the immunoprecipitation using an anti-myc antibody. The input lane represents 1/10 of the cell lysate used for immunoprecipitation.
FIG. 7.
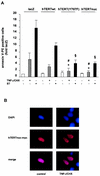
Nuclear localization of hTERT enhances its antiapoptotic function. (A) 293 cells were transfected with LacZ, hTERTwt, hTERT(Y707F), or hTERTnuc and incubated with 10 μM staurosporine (ST) or 100 ng of TNF-α per ml and 10 μg of CHX per ml (TNF-α/CHX) for 3 h. Apoptosis was measured with annexin V by FACS analysis. *, P < 0.01 versus LacZ plus TNF-α/CHX; #, P < 0.05 versus hTERTwt plus TNF-α/CHX; §, P < 0.05 versus hTERTwt plus staurosporine (n = 4 to 6). (B) 293 cells were transfected with hTERTnuc and then incubated with TNF-α and cycloheximide for 3 h. A representative immunostaining is shown (n = 5). (Upper panels) Staining of nuclei with DAPI (blue). (Middle panels) hTERTnuc-myc staining (red). The lower panels show the merge.
Similar articles
- Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase.
Jakob S, Schroeder P, Lukosz M, Büchner N, Spyridopoulos I, Altschmied J, Haendeler J. Jakob S, et al. J Biol Chem. 2008 Nov 28;283(48):33155-61. doi: 10.1074/jbc.M805138200. Epub 2008 Oct 1. J Biol Chem. 2008. PMID: 18829466 Free PMC article. - Downregulation of mitochondrial telomerase reverse transcriptase induced by H2O2 is Src kinase dependent.
Büchner N, Zschauer TC, Lukosz M, Altschmied J, Haendeler J. Büchner N, et al. Exp Gerontol. 2010 Aug;45(7-8):558-62. doi: 10.1016/j.exger.2010.03.003. Epub 2010 Mar 6. Exp Gerontol. 2010. PMID: 20211239 - Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells.
Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Haendeler J, et al. Circ Res. 2004 Apr 2;94(6):768-75. doi: 10.1161/01.RES.0000121104.05977.F3. Epub 2004 Feb 12. Circ Res. 2004. PMID: 14963003 - Nuclear Functions of the Tyrosine Kinase Src.
Bagnato G, Leopizzi M, Urciuoli E, Peruzzi B. Bagnato G, et al. Int J Mol Sci. 2020 Apr 11;21(8):2675. doi: 10.3390/ijms21082675. Int J Mol Sci. 2020. PMID: 32290470 Free PMC article. Review.
Cited by
- Telomere lengthening and other functions of telomerase.
Rubtsova MP, Vasilkova DP, Malyavko AN, Naraikina YV, Zvereva MI, Dontsova OA. Rubtsova MP, et al. Acta Naturae. 2012 Apr;4(2):44-61. Acta Naturae. 2012. PMID: 22872811 Free PMC article. - HIV-1 induces telomerase activity in monocyte-derived macrophages, possibly safeguarding one of its reservoirs.
Reynoso R, Wieser M, Ojeda D, Bönisch M, Kühnel H, Bolcic F, Quendler H, Grillari J, Grillari-Voglauer R, Quarleri J. Reynoso R, et al. J Virol. 2012 Oct;86(19):10327-37. doi: 10.1128/JVI.01495-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787205 Free PMC article. - Telomere shortening may be associated with human keloids.
De Felice B, Wilson RR, Nacca M. De Felice B, et al. BMC Med Genet. 2009 Oct 28;10:110. doi: 10.1186/1471-2350-10-110. BMC Med Genet. 2009. PMID: 19863817 Free PMC article. - Diverse regulatory manners of human telomerase reverse transcriptase.
Jie MM, Chang X, Zeng S, Liu C, Liao GB, Wu YR, Liu CH, Hu CJ, Yang SM, Li XZ. Jie MM, et al. Cell Commun Signal. 2019 Jun 11;17(1):63. doi: 10.1186/s12964-019-0372-0. Cell Commun Signal. 2019. PMID: 31186051 Free PMC article. Review. - Human telomerase activity regulation.
Wojtyla A, Gladych M, Rubis B. Wojtyla A, et al. Mol Biol Rep. 2011 Jun;38(5):3339-49. doi: 10.1007/s11033-010-0439-x. Epub 2010 Nov 18. Mol Biol Rep. 2011. PMID: 21086176 Free PMC article. Review.
References
- Blackburn, E. H. 2000. Telomere states and cell fates. Nature 408:53-56. - PubMed
- Blasco, M. A. 2002. Telomerase beyond telomeres. Nat. Rev. Cancer 2:627-633. - PubMed
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. - PubMed
- Breitschopf, K., A. M. Zeiher, and S. Dimmeler. 2001. Proatherosclerotic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 493:21-25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous