Glycogen synthase kinase 3beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling - PubMed (original) (raw)
Glycogen synthase kinase 3beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling
Joseph F Sanchez et al. Mol Cell Biol. 2003 Jul.
Abstract
Recent studies have revealed a positive correlation between astrocyte apoptosis and rapid disease progression in persons with neurodegenerative diseases. Glycogen synthase kinase 3beta (GSK-3beta) is a molecular regulator of cell fate in the central nervous system and a target of the phosphatidylinositol 3-kinase (PI-3K) pathway. We have therefore examined the role of the PI-3K pathway, and of GSK-3beta, in regulating astrocyte survival. Our studies indicate that inhibition of PI-3K leads to apoptosis in primary cortical astrocytes. Furthermore, overexpression of a constitutively active GSK-3beta mutant (S9A) is sufficient to cause astrocyte apoptosis, whereas an enzymatically inactive GSK-3beta mutant (K85M) has no effect. In light of reports on the interplay between GSK-3beta and nuclear factor kappaB (NF-kappaB), and because of the antiapoptotic activity of NF-kappaB, we examined the effect of GSK-3beta overexpression on NF-kappaB activation. These experiments revealed strong inhibition of NF-kappaB activation in astrocytes upon overexpression of the S9A, but not the K85M, mutant of GSK-3beta. This was accompanied by stabilization of the NF-kappaB-inhibitory protein, IkappaBalpha and down-regulation of IkappaB kinase (IKK) activity. These findings therefore implicate GSK-3beta as a regulator of NF-kappaB activation in astrocytes and suggest that the pro-apoptotic effects of GSK-3beta may be mediated at least in part through the inhibition of NF-kappaB pathway.
Figures
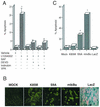
FIG. 1.
Inhibition of PI-3K activity leads to GSK-3β-dependent cell death in primary astrocytes. (A) Astrocytes treated with 50 μM LY294002 for 24 h were fixed and cellular apoptosis was quantitated using TUNEL assay method, revealing over 20% apoptotic nuclei (20.75% ± 3.45%). Alternatively, cells were coincubated with general caspase inhibitors (BAF, 50 μM, or Z-DEVD-FMK, 20 μM), both of which reduced apoptosis to control levels. Cells were also incubated with LY294002 in the presence of inhibitors of GSK-3β activity, such as 0.2 μM Indirubin-3′-monoxime or 1 mM VPA; these reagents also significantly inhibited cell death. The results are shown as mean numbers of apoptotic astrocytes, plus or minus the standard error of the mean values; data were calculated from three independent experiments. *, statistically significant increase in cell death, relative to vehicle-treated cells (P < 0.008; Student's paired t test). (B) Relative expression of adenovirus vector-encoded proteins in astrocytes. Cells were mock infected (no adenovirus [mock]) or infected with the indicated adenovirus vectors at an MOI of 10 (vectors used were Ad-HA-GSK3β K85M [K85M], Ad-HA-GSK3β S9A [S9A], Ad-LacZ [LacZ], and Ad-mIκBα [mIκBα]). Twenty-four hours following virus infection, the expression of exogenous proteins was examined in the cell cultures, by performing either histochemical staining (LacZ), or indirect immunofluorescence analysis using antibodies specific either for the HA epitope tag (K85M, S9A) or for the carboxy terminus of IκBα (mIκBα). As the panels show, approximately 90 to 100% of the cells in the culture were positive for the virally encoded exogenous proteins. Note that the very intense, punctate staining detected in the S9A and mIκBα panels, is due to the presence of apoptotic cell bodies in these cultures. (C) Quantitative analysis of cell death as determined by staining of cell cultures, using the TUNEL assay. Exposure of cells to Ad-mIκBα or to Ad-HA-GSK3β S9A resulted in extensive cell death (66.0% ± 1.0% or 27.5% ± 2.5% of cells, respectively), whereas exposure of cells to Ad-HA-GSK3β K85M or Ad-LacZ resulted in levels of cell death that were not statistically different from those detected in mock-infected cells. The results are shown represent mean values, ± the standard error of the mean (SEM), calculated from three independent experiments. *, statistically significant increase in cell death, relative to vehicle-treated cells (P < 0.02; Student's paired t test).
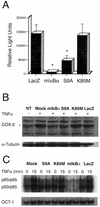
FIG. 2.
GSK-3β overexpression blocks endogenous NF-κB activation at the levels of transcription and DNA binding. (A) Luciferase activity was measured in astrocytes that were transfected with a NF-κB-luciferase reporter plasmid and then infected with adenovirus vectors encoding the indicated GSK-3β isoforms (S9A, K85M), mIκBα, or a control protein, LacZ in the presence of the general apoptosis inhibitor, BAF (50 μM). Cells were treated with recombinant rat TNF-α (20 ng/ml) for 5 h prior to lysis and measurement of luciferase activity. The basal luciferase activity in vehicle-treated cells expressing the control protein LacZ was noted as 4,000 light units (not shown), whereas treatment of these cells with TNF-α led to a >3-fold induction of NF-κB-dependent luciferase activity. Overexpression of mIκBα resulted in complete inhibition of NF-κB-dependent luciferase expression. Similarly, expression of the constitutively active GSK-3β mutant (S9A), but not of the inactive mutant (K85M), resulted in a substantial (approximately threefold) reduction in luciferase activity when compared to control conditions. In this figure, all data for firefly luciferase activity (NF-κB-luc reporter plasmid) were normalized to sea pansyluciferase activity in the same extract (sea pansy luciferase was expressed from a cotransfected control plasmid that was used as an internal control for transfection efficiency; this plasmid contains the Renilla luciferase gene, cloned immediately downstream of the constitutively active HSV-1 TK promoter). The experiment shown was conducted in triplicate and is representative of three independent experiments that yielded similar results. *, statistically significant decrease in NF-κB-dependent luciferase activity, relative to control (LacZ) conditions (P < 0.02; student's paired t test). (B) GSK-3β represses COX-2 expression. Astrocytes were infected with the indicated adenovirus vectors (MOI = 10) or mock infected (NT, Mock) in the presence of the general apoptosis inhibitor BAF (50 μM). Six hours later, the cultures were treated with vehicle alone (NT, −) or exposed to recombinant rat TNF-α (40 ng/ml) for an additional 6 h (+), and cells were then harvested. Lysates containing equivalent amounts of total protein (as determined by Bradford assay) were subjected to immunoblot analysis using antisera specific for the indicated proteins. The top panel (COX-2) illustrates total COX-2 protein levels detected in the lysates and reveals that TNF-α treatment for 6 h results in a significant increase in the expression of endogenous COX-2, except in the case of the Ad-mIκBα- or Ad-HA-GSK3β S9A-infected cultures. The lower panel (α-Tubulin) reveals the level of an irrelevant protein (control) α-tubulin; this provides a loading control for the COX-2 analysis. Expression of exogenous proteins (mIκBα, HA-GSK3β S9A and HA-GSK3β K85M, LacZ) was verified by immunoblot analysis (not shown). The results are representative of two independent experiments. (C) Cells were infected with the indicated adenovirus vectors (MOI = 10), and 6 h later, the cultures were treated with 40 ng of recombinant rat TNF-α per ml for 15 min. Cells were harvested either immediately prior to (lanes 0), or following (lanes 15), addition of TNF-α. EMSA analysis of NF-κB DNA binding activity present in isolated nuclei harvested from these astrocytes was then performed, and the results are shown. Overexpression of the constitutively active S9A mutant of GSK-3β, but not the enzymatically inert GSK-3β mutant (K85M) or an irrelevant control protein (LacZ), resulted in a profound reduction in TNF-α-induced NF-κB DNA binding activity when compared to control conditions (top panel). As expected, expression of mIκBα inhibited basal as well as inducible DNA binding activity of NF-κB. In contrast, none of the conditions or vectors had any effect on the nuclear DNA binding activity for a control transcription factor, Oct-1 (lower panel_)_. The data shown are representative of three independent experiments.
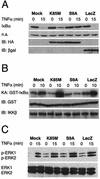
FIG. 3.
Overexpression of a constitutively active GSK-3β mutant results in stabilization of endogenous IκBα. (A) Cells were infected with the indicated adenovirus vectors (MOI = 10), and 6 h later, the cultures were treated with recombinant rat TNF-α (40 ng/ml) for 15 min. Cells were harvested either immediately prior to (lanes 0) or following (lanes 15) addition of TNF-α and lysates containing equivalent amounts of total protein (as determined by Bradford assay) were then subjected to immunoblot analysis using various antisera. The top panel (IκBα) illustrates total IκBα protein levels detected in the lysates and reveals that TNF-α treatment for 15 min results in extensive degradation of endogenous IκBα, except in the case of the S9A-infected cultures. The second panel (n.s.) reveals the level of an irrelevant protein of unknown identity that was also reactive with the IκBα-specific antiserum; this provides a loading control for the IκBα analysis. The bottom two panels (IB:HA and IB:βgal) show, respectively, the results of immunoblot analyses that were conducted using monoclonal antibodies specific for the HA epitope tag (present on both of the GSK-3β mutants) and β-galactosidase (expressed by Ad-LacZ); these data reveal abundant overexpression of each of the rAd-encoded proteins at 6 h following infection of the astrocyte cultures. All panels in this figure show results from representative experiments performed at least three times with similar results. (B) In vitro kinase assay for activity of endogenous IKK. In this analysis, cells were infected with the indicated adenovirus vectors (MOI = 10), and 6 h later, cultures were harvested for analysis (lanes 0) or treated with recom-binant rat TNF-α for 10 min (lanes 10) to initiate NF-κB signaling and activation of endogenous IKK. Cell lysates were then prepared, and IKKβ-containing IKK complexes were immunoprecipitated using a specific antibody and protein A beads. The immunoprecipitated IKK was then mixed with substrate protein (GST-IκBα) and [γ-32P]ATP in order to perform a kinase assay for IKK activity. The top panel (KA: GST-IκBα) shows the level of radiolabeled GST-IκBα that was generated in these kinase assays. The panel immediately below this (IB:GST) shows the result of an immunoblot assay using a GST-specific antibody, performed on the same samples (a control to verify the presence of the substrate protein in all of the reactions). Finally, the bottom panel (IB:IKKβ) shows the result of an immunoblot assay using an IKKβ-specific antibody, also performed on protein A beads from the same samples (a control to verify that equivalent amounts of IKKβ protein were present in all of the reactions). The data show that overexpression of the GSK-3β S9A mutant results in the inhibition of TNF-α-induced IKK activity in primary astrocytes. (C) Analysis of ERK kinase activity. In these experiments, all cultures were treated as described above. Cell lysates were then prepared and subjected to immunoblot analysis using antibodies specific for phosphorylated isoforms of ERK1 and ERK2 (upper panel) or total ERK1 and ERK2 (lower panel). It is apparent that treatment of astrocytes with TNF-α results in the phosphorylation (activation) of ERK1 and ERK2 and that the level of ERK activation is equivalent in all rAd-infected cultures, regardless of the exogenous gene product expressed (in all cases, this is substantially higher than in the mock-infected cells, due to the activating effect of rAd infection on ERK). All panels in this figure show results from representative experiments that were performed twice with similar results.
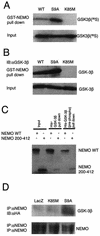
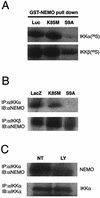
FIG. 4.
Binding of GSK-3β to NEMO. (A) Pull down analysis was performed using GST-NEMO and IVTT radiolabeled GSK-3β proteins (wild type, S9A, and K85M). The radiolabeled IVTT proteins (5 μl) were precleared by incubating with GST-bound glutathione-conjugated Sepharose beads and then allowed to bind to equal amount of GST-NEMO (1 μg), prebound to the glutathione-conjugated Sepharose beads. The binding reaction was performed at 4°C with rotation for 1 h. After extensive washing of the beads, the proteins were elutedin sample buffer and fractionated by SDS-10% PAGE and analyzed by autoradiography. The top panel (lanes GST-NEMO pull down) shows the level of radiolabeled GSK-3β that was bound to NEMO. The lower panel (lanes Input) shows 10% of input that was used in these assays (a control to verify that equal amounts of radiolabeled GSK-3β proteins were present in all the reactions). (B) Experiments analogous to those in panel A were performed in which proteins bound to GST-NEMO were subjected to immunoblot analysis using GSK-3β-specific antibodies. (C) Reciprocal experiments were performed in which recombinant protein His-GSK-3β prebound to TALON metal affinity resin was allowed to bind IVTT-derived radiolabeled NEMO (either wild type or truncated mutant [residues 200 to 412]). In some experiments, His-GSK-3β was prephosphorylated by using purified PKA and cold ATP which was then used in pull down assays [lanes His-GSK-3β (pre-phosph) pull down]. The bound proteins were analyzed by SDS-PAGE and autoradiography. (D) Binding of GSK-3β to NEMO was analyzed using extracts of astrocytes. In this experiment, astrocytes were infected with the indicated rAd vectors (LacZ, K85M, S9A) in the presence of BAF (50 μM); 12 h later whole-cell lysates were prepared, and endogenous NEMO was immunoprecipitated using specific antibody and protein G-agarose beads. The immune complexes were then subjected to immunoblot analysis using either anti-HA (upper panel; to analyze the presence of HA-tagged proteins in the complex) or anti-NEMO (lower panel; a control to analyze the presence of NEMO in the complex) antibodies. All panels in this figure show results from representative experiments that were performed twice with similar results.
FIG. 5.
GSK-3β inhibits binding of IKK to NEMO. (A) Equal amounts of IVTT-derived proteins corresponding to either nonradiolabeled (luciferase-Luc; GSK-3β K85M and S9A) or radiolabeled IKKα (upper panel) or IKKβ (lower panel) were mixed and allowed to bind to a limited amount of GST-NEMO (1 μg) prebound to glutathione-Sepharose beads. The beads were then used for pull down analysis, followed by SDS-PAGE and autoradiography. The result shows that the presence of GSK-3β S9A, but not Luc (irrelevant control) or GSK-3β K85M, abolishes the binding of IKK (α and β isoforms) to NEMO. (B) Astrocytes were infected by using rAd vectors to express the indicated proteins in the presence of BAF (50 μM) for 12 h. Immunoprecipitation and immunoblot analysis of the whole cell extract was performed, as described in Fig. 4D, using the indicated antibodies. In this case, overexpression of GSK-3β was able to block the interaction of IKKα (upper panel) or IKKβ (lower panel) with NEMO. (C) Astrocytes were either left alone (lane NT) or treated with PI-3K inhibitor LY294002 (50 μM; lane LY) for 4 h. The cells were then incubated with 40 ng/ml recombinant rat TNF-α for 10 min. After this, whole-cell extracts were prepared and analyzed by performing immunoprecipitation and immunoblot analysis using the indicated antibodies. All the results shown in this figure are representative of two independent experiments.
Similar articles
- Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor.
Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Takada Y, et al. J Biol Chem. 2004 Sep 17;279(38):39541-54. doi: 10.1074/jbc.M403449200. Epub 2004 Jul 13. J Biol Chem. 2004. PMID: 15252041 - Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte.
Kim SD, Yang SI, Kim HC, Shin CY, Ko KH. Kim SD, et al. Brain Res. 2007 Dec;1186:12-20. doi: 10.1016/j.brainres.2007.10.018. Epub 2007 Oct 18. Brain Res. 2007. PMID: 17996850 - Primers on molecular pathways. The glycogen synthase kinase-3beta.
Billadeau DD. Billadeau DD. Pancreatology. 2007;7(5-6):398-402. doi: 10.1159/000108955. Epub 2007 Oct 1. Pancreatology. 2007. PMID: 17912008 Free PMC article. Review. - Association of glycogen synthase kinase-3β with Parkinson's disease (review).
Li DW, Liu ZQ, Chen W, Yao M, Li GR. Li DW, et al. Mol Med Rep. 2014 Jun;9(6):2043-50. doi: 10.3892/mmr.2014.2080. Epub 2014 Mar 28. Mol Med Rep. 2014. PMID: 24681994 Free PMC article. Review.
Cited by
- Ellagic acid coordinately attenuates Wnt/β-catenin and NF-κB signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis.
Anitha P, Priyadarsini RV, Kavitha K, Thiyagarajan P, Nagini S. Anitha P, et al. Eur J Nutr. 2013 Feb;52(1):75-84. doi: 10.1007/s00394-011-0288-y. Epub 2011 Dec 11. Eur J Nutr. 2013. PMID: 22160170 - GSK-3β: A Bifunctional Role in Cell Death Pathways.
Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. Jacobs KM, et al. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. Epub 2012 May 21. Int J Cell Biol. 2012. PMID: 22675363 Free PMC article. - Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation.
Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Lee JY, et al. Mol Cell Biol. 2007 Apr;27(8):3187-98. doi: 10.1128/MCB.01461-06. Epub 2007 Feb 5. Mol Cell Biol. 2007. PMID: 17283049 Free PMC article. - Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals.
Davidson DC, Hirschman MP, Spinelli SL, Morrell CN, Schifitto G, Phipps RP, Maggirwar SB. Davidson DC, et al. J Immunol. 2011 Jan 1;186(1):584-91. doi: 10.4049/jimmunol.1001911. Epub 2010 Nov 29. J Immunol. 2011. PMID: 21115729 Free PMC article. - Novel dual-reporter preclinical screen for antiastrocytoma agents identifies cytostatic and cytotoxic compounds.
Hawes JJ, Nerva JD, Reilly KM. Hawes JJ, et al. J Biomol Screen. 2008 Sep;13(8):795-803. doi: 10.1177/1087057108321085. Epub 2008 Jul 29. J Biomol Screen. 2008. PMID: 18664715 Free PMC article.
References
- Abbott, K. L., A. M. Robida, M. E. Davis, G. K. Pavlath, J. M. Camden, J. T. Turner, and T. J. Murphy. 2000. Differential regulation of vascular smooth muscle nuclear factor kappa-B by G alpha q-coupled and cytokine receptors. J. Mol. Cell. Cardiol. 32:391-403. - PubMed
- Allport, V. C., D. M. Slater, R. Newton, and P. R. Bennett. 2000. NF-κB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol. Hum. Reprod. 6:561-565. - PubMed
- Barbin, G., M. P. Roisin, and B. Zalc. 2001. Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons. Neurochem. Res. 26:107-112. - PubMed
- Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. - PubMed
- Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-alpha-induced cell death. Science 274:782-784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS40315/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- R01 NS39039/NS/NINDS NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 MH056838/MH/NIMH NIH HHS/United States
- R01 MH56838/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous