Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter - PubMed (original) (raw)
Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter
Tim Veldman et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The papillomavirus E6 protein binds and directs the ubiquitin-dependent degradation of the p53 tumor suppressor protein. Independent of this p53-degradative function, however, E6 induces cellular telomerase activity. This increase in enzyme activity reflects E6-enhanced transcription of the human telomerase reverse transcriptase (hTERT) catalytic subunit, but the molecular basis for this transactivation is unknown. In the present study, we demonstrate that E6/Myc interactions regulate hTERT gene expression. Mad protein, a specific antagonist of Myc, repressed E6-mediated transactivation of the hTERT promoter and this repression was relieved by Myc overexpression. The proximal Myc/ Max-binding element (E-box) in the hTERT promoter was the major determinant of both E6 and Myc responsiveness in keratinocytes. E6 did not alter Myc protein expression or Myc/Max association, and the induction of hTERT by Myc/E6 was independent of Myc phosphorylation at Thr-58/Ser-62 within the transactivation domain. However, immunoprecipitation studies demonstrated that endogenous Myc protein coprecipitated with E6 protein and chromatin immunoprecipitation analyses demonstrated that both E6 and Myc proteins bound to a minimal 295-bp hTERT promoter. Only the "high-risk" E6 proteins bound to the hTERT promoter, consistent with their preferential ability to induce telomerase. The observation that E6 associates with Myc complexes and activates a Myc-responsive gene identifies a mechanism by which this oncogene can modulate cell proliferation and differentiation.
Figures
Fig. 1.
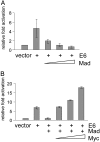
E6-mediated activation of the hTERT promoter is repressed by Mad and restored by Myc. (A) Increasing amounts of Mad plasmid (2, 10, and 50 ng) inhibit E6-induced hTERT-promoter activity. A 295-bp hTERT-promoter fragment (+40 to -255) was cloned into the pGL3-Basic vector (Promega) upstream of the firefly luciferase reporter gene as described (34). The resulting construct (pGL3B-255, see Fig. 2_A_) was transfected into keratinocytes with an E6 expression vector, the pRL-cytomegalovirus R. reniformis reporter plasmid (to standardize transfection efficiencies), and increasing amounts of the Mad plasmid. Relative fold activation represents the normalized activity of pGL3B-255 plus empty vector compared to the normalized activity induced by the indicated expression plasmids. (B) Increasing amounts of Myc plasmid (2, 10, and 50 ng) restore E6-induced hTERT-promoter activity. The amount of the E6 and Mad plasmids was kept constant (10 ng of each) in each transfection. Error bars represent the SD of at least three independent experiments.
Fig. 3.
E6 does not require Myc protein phosphorylation at Thr-58/Ser-62 to induce hTERT promoter activity. (A) Domains of the Myc protein and location of two major phosphorylation sites (○). The major phosphorylation sites of Myc, Thr-58, and Ser-62, were replaced with Ala residues by site-directed mutagenesis (•). TAD, transactivation domain; NLS, nuclear localization signal; b, basic domain; HLH, helix–loop–helix domain; LZ, luceine zipper domain. (B) Mutant Myc proteins are phosphorylation-defective. WT and mutant Myc expression proteins were transiently expressed in Cos cells, and Western blot analysis was performed by using a Thr-58/Ser-62 phospho-c-Myc-specific Ab (Cell Signaling). HeLa and HFK served as positive controls and IMR-90 as a negative control. Phospho-Myc blots were stripped and reprobed for total Myc to demonstrate equal expression and loading. (C) Phosphorylation-defective Myc proteins still cooperate with E6 in the induction of the hTERT promoter. Transient reporter assays were performed as in Fig. 2. The hTERT promoter was cotransfected with the E6 and Myc constructs as indicated. Error bars represent the SD of at least three independent experiments.
Fig. 2.
An intact downstream E-box is required for activation of the hTERT promoter by either E6 or Myc. (A) Schematic representation of the WT and mutant hTERT constructs. The WT hTERT core promoter (pGL3B-255) was mutated at either or both of the E-box elements (Myc-binding sites). Double-point mutations were introduced at each of these sites to generate an upstream mutant (pGL3B-Up), downstream mutant (pGL3B-Dn), or double mutant (pGL3B-BM). The E-box locations are -186 and +22 nt from the transcription start site. Checkered boxes (-CACGTG-) represent the WT E-boxes, black boxes represent the mutant E-boxes, and black bars represent the location of primers. (B) Effect of E-box mutations on E6-induced hTERT-promoter activity. The WT and mutant hTERT promoters were evaluated for transcriptional activity after introduction of the E6 gene (or vector control). Transient reporter assays were performed as described in Fig. 1. (C) Effect of E-box mutations on Myc-induced hTERT-promoter activity. The same protocol as for B except that the Myc gene was transfected with the hTERT-promoter constructs. Error bars represent the SD of at least three independent experiments.
Fig. 4.
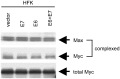
E6 does not alter the levels of Myc–Max heterocomplexes. Proteins were extracted from exponentially growing, transduced HFK cells as described (50) and then IP and IB with either anti-Myc or anti-Max Ab. (Top) Total c-Max expression detected by anti-Max IP and IB. (Bottom) Total c-Myc expression detected by anti-Myc IP and IB. (Middle) The fraction of c-Myc protein complexed with c-Max protein detected by anti-Max IP and anti-Myc IB.
Fig. 5.
E6 binds to Myc complexes and the hTERT promoter in vivo.(A) Coimmunoprecipitation of E6 and endogenous Myc proteins in stably transduced HFK cells. Protein lysates of E6- and E7-transduced cells were immunoprecipitated with Myc, Max, or E6 Abs, and the precipitates were immunoblotted with E6 or Myc Ab. As anticipated, Myc protein was detectable by IB in both Myc and Max immunoprecipitates. Myc was also detectable in E6-immunoprecipitates (using Abs against E6 protein), but only in those cells expressing the E6 gene and not in those expressing E7. (B) Coimmunoprecipitation of epitope-tagged E6 protein with exogenous Myc in transiently transfected COS cells. COS cells were transfected with the indicated plasmid constructs and analyzed by IP/IB as described in A. The E6 protein was immunoprecipitated with AU1 Ab, which reacts with an appended 6-aa epitope at the E6 C terminus. Myc was detectable in AU1 immunoprecipitates only when E6-AU1 was present. (C) CHIP assay detects Myc and high-risk E6 proteins bound to an exogenous hTERT promoter. (Left) HFK cells were transiently cotransfected with the pGL3B-255 hTERT plasmid, Myc plasmid, and HPV-16 E6 plasmid. After precipitation of protein–DNA complexes with anti-E6 or anti-Myc Ab, PCR amplification was performed by using hTERT-promoter primers (416-bp product). A positive signal was observed with the anti-Myc and anti-E6 Abs, but not with the control rabbit and goat Abs. (Center) Vector-transfected cells failed to give a signal with anti-E6 or anti-Myc Abs. (Right) HFK cells were transfected with the hTERT and Myc plasmids plus either the AU1-tagged HPV-16 or HPV-6b E6 genes. IP was performed with the AU1 Ab. Only the high-risk HPV-16 E6 protein bound the hTERT promoter. Input refers to DNA from transfected HFK cells. (D) CHIP assay detects E6 and Myc proteins bound to the endogenous hTERT promoter. E6 or Myc Ab was used to precipitate endogenous protein–DNA complexes from stable E6- or E7-expressing cells. The complexes were then subjected to PCR amplification to generate a 257-bp product of the hTERT promoter. The anti-E6 Ab generated a positive signal in E6- but not E7-expressing cells. Myc was associated with the hTERT promoter in both E6- and E7-expressing cells.
Similar articles
- The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein.
Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. Liu X, et al. J Biol Chem. 2005 Mar 18;280(11):10807-16. doi: 10.1074/jbc.M410343200. Epub 2005 Jan 17. J Biol Chem. 2005. PMID: 15655249 - Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc.
Liu X, Dakic A, Chen R, Disbrow GL, Zhang Y, Dai Y, Schlegel R. Liu X, et al. J Virol. 2008 Dec;82(23):11568-76. doi: 10.1128/JVI.01318-08. Epub 2008 Sep 25. J Virol. 2008. PMID: 18818322 Free PMC article. - Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms.
Horikawa I, Barrett JC. Horikawa I, et al. Carcinogenesis. 2003 Jul;24(7):1167-76. doi: 10.1093/carcin/bgg085. Epub 2003 May 22. Carcinogenesis. 2003. PMID: 12807729 Review. - Activation of telomerase by HPVs.
Katzenellenbogen RA. Katzenellenbogen RA. Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
Cited by
- Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer.
Faraji F, Zaidi M, Fakhry C, Gaykalova DA. Faraji F, et al. Microbes Infect. 2017 Sep-Oct;19(9-10):464-475. doi: 10.1016/j.micinf.2017.06.001. Epub 2017 Jun 12. Microbes Infect. 2017. PMID: 28619685 Free PMC article. Review. - Endometriosis Is Associated with a Significant Increase in hTERC and Altered Telomere/Telomerase Associated Genes in the Eutopic Endometrium, an Ex-Vivo and In Silico Study.
Alnafakh R, Choi F, Bradfield A, Adishesh M, Saretzki G, Hapangama DK. Alnafakh R, et al. Biomedicines. 2020 Dec 9;8(12):588. doi: 10.3390/biomedicines8120588. Biomedicines. 2020. PMID: 33317189 Free PMC article. - The large and small isoforms of human papillomavirus type 16 E6 bind to and differentially affect procaspase 8 stability and activity.
Filippova M, Johnson MM, Bautista M, Filippov V, Fodor N, Tungteakkhun SS, Williams K, Duerksen-Hughes PJ. Filippova M, et al. J Virol. 2007 Apr;81(8):4116-29. doi: 10.1128/JVI.01924-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267478 Free PMC article. - The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation.
Scarth JA, Patterson MR, Morgan EL, Macdonald A. Scarth JA, et al. J Gen Virol. 2021 Mar;102(3):001540. doi: 10.1099/jgv.0.001540. Epub 2021 Jan 11. J Gen Virol. 2021. PMID: 33427604 Free PMC article. Review. - The human papillomavirus 16 E6 protein can either protect or further sensitize cells to TNF: effect of dose.
Filippova M, Brown-Bryan TA, Casiano CA, Duerksen-Hughes PJ. Filippova M, et al. Cell Death Differ. 2005 Dec;12(12):1622-35. doi: 10.1038/sj.cdd.4401678. Epub 2005 Jun 3. Cell Death Differ. 2005. PMID: 15933739 Free PMC article.
References
- Greider, C. W. (1996) Annu. Rev. Biochem. 65 337-365. - PubMed
- Watson, J. D. (1972) Nat. New Biol. 239 197-201. - PubMed
- Harley, C. B., Futcher, A. B. & Greider, C. W. (1990) Nature 345 458-460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA106400/CA/NCI NIH HHS/United States
- R01 CA53371/CA/NCI NIH HHS/United States
- F31 CA090203/CA/NCI NIH HHS/United States
- R01 CA053371/CA/NCI NIH HHS/United States
- 1 F31 CA90203-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous