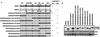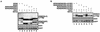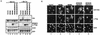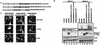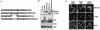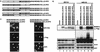Critical contribution of the MDM2 acidic domain to p53 ubiquitination - PubMed (original) (raw)
Critical contribution of the MDM2 acidic domain to p53 ubiquitination
Hidehiko Kawai et al. Mol Cell Biol. 2003 Jul.
Abstract
MDM2 is an E3 ubiquitin ligase that targets p53 for proteasomal degradation. Recent studies have shown, however, that the ring-finger domain (RFD) of MDM2, where the ubiquitin E3 ligase activity resides, is necessary but not sufficient for p53 ubiquitination, suggesting that an additional activity of MDM2 might be required. To test this possibility, we generated a series of MDM2/MDMX chimeric proteins to assess the contribution of each domain of MDM2 to the ubiquitination process. MDMX is a close structural homolog of MDM2 that nevertheless lacks the E3 ligase activity in vivo. We demonstrate here that MDMX gains self-ubiquitination activity and becomes extremely unstable upon introduction of the MDM2 RFD, indicating that the RFD is essential for self-ubiquitination. This MDMX chimeric protein, however, is unable to ubiquitinate p53 in vivo despite its E3 ligase activity and binding to p53, separating the self-ubiquitination activity of MDM2 from its ability to ubiquitinate p53. Significantly, fusion of the central acidic domain (AD) of MDM2 to the MDMX chimeric protein renders the protein fully capable of ubiquitinating p53, and p53 ubiquitination is associated with p53 degradation and nuclear export. Moreover, the AD mini protein expressed in trans can functionally rescue the AD-lacking MDM2 mutant, further supporting a critical role for the AD in MDM2-mediated p53 ubiquitination.
Figures
FIG. 1.
MDM2/MDMX chimeras. (A) MDM2/MDMX chimeras were generated by swapping corresponding domains between MDM2 and MDMX at the indicated positions by using a two-step PCR with primers carrying the 12-nucleotide overlap at the parts to be fused. NES, nuclear export sequence; Nuc., nucleus; Cyto., cytoplasm. (B) cDNAs encoding the indicated chimeras were subcloned into pCMV-Flag expression vector, which was then transfected into p53−/−/MDM2−/− MEFs with enhanced GFP (EGFP)-empty vector (0.2 μg) included as a transfection efficiency control, and the transfectants were harvested at 36 h posttransfection. Whole-cell extracts were prepared, boiled in loading dye, resolved on SDS-PAGE, and transferred onto a nitrocellulose membrane. The membrane was then probed with anti-Flag, anti-GFP, or anti-actin antibody.
FIG. 2.
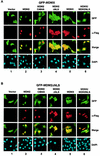
Subcellular distribution of the MDM2/MDMX complexes. (A) EGFP-MDMX expression vector was cotransfected with the indicated Flag-tagged vector into p53−/−/MDM2−/− MEFs. The transfectants were fixed at 36 h posttransfection and stained with an anti-Flag antibody and secondary antibody (Texas Red X-goat anti-mouse immunoglobulin G). Direct green fluorescence and Texas Red positive staining were visualized under a fluorescent microscope. Cellular nuclei were identified by DAPI staining. Merged images were obtained by superimposing GFP over Texas Red staining. (B) EGFP-MDM2ΔNLS expression vector was coexpressed with the indicated Flag vector in p53−/−/MDM2−/− MEFs, and cells were analyzed as described for panel A.
FIG. 3.
The MDM2 RFD is necessary and sufficient for self-ubiquitination activity. p53−/−/MDM2−/− MEFs were transfected with the indicated plasmids (2 μg) encoding MDM2, MDMX, or an MDM2/MDMX chimera and EGFP-empty vector (0.2 μg). Cells were treated with MG132 (7.5 μg/ml) or left untreated at 30 h posttransfection and incubated for an additional 6 h before being harvested for Western analysis by using the indicated antibodies. LE, long exposure; SE, short exposure.
FIG. 4.
The MDM2 RFD is not sufficient for p53 ubiquitination. (A) p53 expression vector (1 μg) was cotransfected with the indicated MDM2/MDMX chimera vector (2 μg) into p53−/−/MDM2−/− MEFs. Wild-type MDM2 or MDMX was included as a control. Cells were treated with MG132 (lanes 6 to 10) or left untreated (lanes 1 to 5) for 6 h and then analyzed as described in the legend to Fig. 3. (B) EGFP-p53 vector (1 μg) was cotransfected with the MDM2/MDMX chimera (2 μg) into p53−/−/MDM2−/− MEFs, and cells were analyzed as described in the legend to Fig. 2.
FIG. 5.
The AD of MDM2 is another essential element for p53 ubiquitination. (A) p53−/−/MDM2−/− MEFs were cotransfected with p53 and the indicated chimeras. The cells were analyzed by Western blotting (B) or immunostaining (C).
FIG. 6.
Both the AD and the RFD of MDM2 are required for p53 ubiquitination. p53−/−/MDM2−/− MEFs were cotransfected with p53 and the indicated vector as shown in panel A. The cells were analyzed by Western blotting (B) or immunostaining (C).
FIG. 7.
The AD of MDM2 collaborates with the RFD in p53 ubiquitination and can be provided in trans. (A) The indicated MDM2/MDMX chimeric protein expression vectors were transfected either alone or as a pair into p53−/−/MDM2−/− MEFs. Cells were analyzed by Western blotting (B) or immunostaining (C).
FIG. 8.
Functional rescue of the AD-lacking mutant of MDM2 by MDM2 AD-containing chimeras. (A) p53−/−/MDM2−/− MEFs were cotransfected with an AD deletion mutant of MDM2 and with the indicated construct. The cells were analyzed by Western blotting (B) or immunostaining (C). V, vector; LE, long exposure; SE, short exposure. (D) Binding of the MDM2 AD (residues 109 to 303) to p53 was analyzed by incubating lysates from cells expressing Flag-MDM2 AD with the indicated GST-p53 deletion proteins. The adsorbents were resolved by SDS-PAGE and stained with Ponceau S solution (top panel) or Western blotted with an anti-Flag antibody (bottom panel). GST-P300/CH1 was included as a positive control. (E) Anti-p53 immunoprecipitations were performed with cell lysates prepared from the indicated constructs transfected into p53−/−/MDM2−/− MEFs. The whole-cell extracts (WCE) and anti-p53 immunocomplexes were analyzed by immunoblotting (IB) with anti-p53 (top two panels) and anti-Flag (bottom four panels). IP, immunoprecipitate; IgG, immunoglobulin G.
Similar articles
- Critical role for a central part of Mdm2 in the ubiquitylation of p53.
Meulmeester E, Frenk R, Stad R, de Graaf P, Marine JC, Vousden KH, Jochemsen AG. Meulmeester E, et al. Mol Cell Biol. 2003 Jul;23(14):4929-38. doi: 10.1128/MCB.23.14.4929-4938.2003. Mol Cell Biol. 2003. PMID: 12832478 Free PMC article. - Overexpression of Mdm2 and MdmX fusion proteins alters p53 mediated transactivation, ubiquitination, and degradation.
Ghosh M, Huang K, Berberich SJ. Ghosh M, et al. Biochemistry. 2003 Mar 4;42(8):2291-9. doi: 10.1021/bi0271291. Biochemistry. 2003. PMID: 12600196 - A site-directed mutagenesis study of the MdmX RING domain.
Egorova O, Mis M, Sheng Y. Egorova O, et al. Biochem Biophys Res Commun. 2014 May 16;447(4):696-701. doi: 10.1016/j.bbrc.2014.04.065. Epub 2014 Apr 19. Biochem Biophys Res Commun. 2014. PMID: 24755078 - p53 regulation: teamwork between RING domains of Mdm2 and MdmX.
Wang X. Wang X. Cell Cycle. 2011 Dec 15;10(24):4225-9. doi: 10.4161/cc.10.24.18662. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22134240 Review. - Mdm2 and MdmX partner to regulate p53.
Wang X, Jiang X. Wang X, et al. FEBS Lett. 2012 May 21;586(10):1390-6. doi: 10.1016/j.febslet.2012.02.049. Epub 2012 Mar 8. FEBS Lett. 2012. PMID: 22673503 Review.
Cited by
- Competitive ubiquitination activates the tumor suppressor p53.
Li X, Guo M, Cai L, Du T, Liu Y, Ding HF, Wang H, Zhang J, Chen X, Yan C. Li X, et al. Cell Death Differ. 2020 Jun;27(6):1807-1818. doi: 10.1038/s41418-019-0463-x. Epub 2019 Dec 2. Cell Death Differ. 2020. PMID: 31796886 Free PMC article. - Intra molecular interactions in the regulation of p53 pathway.
Chen J. Chen J. Transl Cancer Res. 2016 Dec;5(6):639-649. doi: 10.21037/tcr.2016.09.23. Transl Cancer Res. 2016. PMID: 30613472 Free PMC article. - HIV-1 viral infectivity factor interacts with TP53 to induce G2 cell cycle arrest and positively regulate viral replication.
Izumi T, Io K, Matsui M, Shirakawa K, Shinohara M, Nagai Y, Kawahara M, Kobayashi M, Kondoh H, Misawa N, Koyanagi Y, Uchiyama T, Takaori-Kondo A. Izumi T, et al. Proc Natl Acad Sci U S A. 2010 Nov 30;107(48):20798-803. doi: 10.1073/pnas.1008076107. Epub 2010 Nov 11. Proc Natl Acad Sci U S A. 2010. PMID: 21071676 Free PMC article. - Critical role for a central part of Mdm2 in the ubiquitylation of p53.
Meulmeester E, Frenk R, Stad R, de Graaf P, Marine JC, Vousden KH, Jochemsen AG. Meulmeester E, et al. Mol Cell Biol. 2003 Jul;23(14):4929-38. doi: 10.1128/MCB.23.14.4929-4938.2003. Mol Cell Biol. 2003. PMID: 12832478 Free PMC article. - Controlling the Mdm2-Mdmx-p53 Circuit.
Waning DL, Lehman JA, Batuello CN, Mayo LD. Waning DL, et al. Pharmaceuticals (Basel). 2010 May 18;3(5):1576-1593. doi: 10.3390/ph3051576. Pharmaceuticals (Basel). 2010. PMID: 20651945 Free PMC article.
References
- Argentini, M., N. Barboule, and B. Wasylyk. 2001. The contribution of the acidic domain of MDM2 to p53 and MDM2 stability. Oncogene 20:1267-1275. - PubMed
- Ashcroft, M., and K. H. Vousden. 1999. Regulation of p53 stability. Oncogene 18:7637-7643. - PubMed
- Boyd, S. D., K. Y. Tsai, and T. Jacks. 2000. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat. Cell Biol. 2:563-568. - PubMed
- Fang, S., J. P. Jensen, R. L. Ludwig, K. H. Vousden, and A. M. Weissman. 2000. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275:8945-8951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous