The human telomere-associated protein TIN2 stimulates interactions between telomeric DNA tracts in vitro - PubMed (original) (raw)
The human telomere-associated protein TIN2 stimulates interactions between telomeric DNA tracts in vitro
Sahn-Ho Kim et al. EMBO Rep. 2003 Jul.
Abstract
Human TIN2 interacts with the telomeric-DNA-binding protein TRF1, suppresses telomere elongation in telomerase-positive cells, and may control telomere length by modulating telomere structure. To test the latter idea, we developed an in vitro assay, using biotinylated telomeric DNA probes and streptavidin-agarose, to quantify the ability of TRF1 and TIN2 to stimulate interactions of telomeric DNA tracts with each other (probe clustering). This assay revealed that TRF1 alone had weak probe-clustering activity, but TIN2 stimulated activity fivefold to tenfold. A dominant-negative TIN2 mutant protein that increased telomere length in vivo disrupted probe clusters formed by TRF1 and TIN2, suggesting that the ability to stimulate telomeric DNA interactions is important for telomere-length regulation. Unlike TRF1, TIN2 did not form homodimers. We propose that TIN2 alters the conformation of TRF1, which favours a tertiary telomeric structure that hinders telomerase from gaining access to telomeres.
Figures
Figure 1
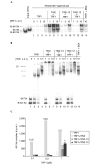
Homotypic TIN2 interactions. (A) TIN2 and TRF1 complementary DNAs were cloned into the yeast vectors pGBT-9, pGAD-10 (Clontech), pTGB-2 or pDAG-2 (Cary et al., 1998), and the indicated vector pairs (1–4) were transformed into yeast. Yeast cells were cultured on selective (HTL+3AT: −His, −Trp, −Leu, plus 10 mM 3-aminotriasol) or nonselective (TL: −Trp, −Leu) media. (B) Amino-terminal FLAG-tagged TIN2 and carboxy-terminal haemagglutinin (HA)-tagged TIN2 were cloned into the pLXSN retroviral vector, and infectious virus was produced and used to infect HT1080 cells, as described in Kim et al. (1999). Lysates alone (lanes 1–4) or mixed with purified His–TRF1 (lanes 5–10) were immunoprecipitated (IP) using anti-FLAG (Sigma) or anti-HA (Santa Cruz) antibodies. The precipitates were analysed by western blotting (WB) using polyclonal anti-HA (Santa Cruz), anti-TRF1, anti-TIN2 or anti-FLAG antibodies, respectively. Ext., cell extract; Gal4-AD, Gal4 activation domain; Gal4-DB, Gal4 DNA-binding domain.
Figure 2
TIN2 proteins. (A) Wild-type TIN2 (amino acids 1–354), TIN2-12 (amino acids 120–354) and TIN2-13 (amino acids 196–354). (B,C) Approximately 2 µg of purified His–TRF1, His–TIN2, His–TIN2-12, His–TIN2-13 and BSA were detected by Coomassie-blue staining and identified by western blotting using an anti-TIN2 antibody. TRF1-Int, TRF1-interacting domain.
Figure 3
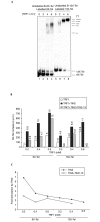
TIN2 stimulates probe clustering. (A) The telomere probes (6X-Tel and B-6X-Tel) used in the assay. (B) Reaction mixtures containing probes (10 µl) and the proteins indicated were analysed by electrophoretic mobility-shift assays. Asterisks indicate TRF1–probe complexes that correspond to the binding of one, two or three TRF1 dimers, as indicated by the number of asterisks. (C) Reaction mixtures (90 µl) in the absence ('buffer') or in the presence of TRF1 (0.25 µM), TIN2 (0.25 µM) or both were captured on streptavidin–agarose beads, released by phenol extraction, precipitated with ethanol, and analysed by native polyacrylamide gel electrophoresis. The B-6X-Tel probe was either labelled (lanes 1–5) or unlabelled (lanes 6–9). For lane 5, excess (100×) unlabelled double-stranded (TTAGGG)7 was added before the addition of proteins ('competitor').
Figure 4
Effect of TRF1 concentration and TIN2 mutant proteins. (A) Reaction mixtures (100 µl) containing 6X-Tel and B-6X-Tel with different concentrations of TRF1 (0.1, 0.3 and 1 µM) were assembled. 1 µM each of TIN2 (lanes 5–7), TIN2-13 (lanes 8–10), TIN2-12 (lanes 11–13) and BSA (control; lane 14) were then added. The captured complexes were released by phenol. After measuring the amount of radioactivity, the released probes were precipitated and analysed by native polyacrylamide gel electrophoresis. (B) 10 µl of the reaction mixtures were analysed by electrophoretic mobility-shift assays. Asterisks indicate TRF1–probe complexes that correspond to the binding of one, two and three TRF1 dimers, as indicated by the number of asterisks. (C) We quantified the amount of probe released by phenol (c.p.m.) by scintillation counting. The amount of stimulation by TIN2 proteins was calculated by dividing the amount of radioactivity released from reactions containing TRF1 and TIN2 by that released from reactions containing only TRF1 ((TRF1+TIN2)/TRF1). Ppt, precipitate; TT, TRF1–probe complex.
Figure 5
Effect of probe length. (A) Labelled 6X-Tel and unlabelled B-6X-Tel (lanes 1–4) or labelled 13X-Tel and unlabelled B-13X-Tel (lane 5–6) probes were incubated with 0, 0.2, 0.4 or 0.6 µM TRF1 and analysed by electrophoretic mobility-shift asssays to detect complexes with increasing numbers (indicated by the number of asterisks) of TRF1 dimers. (B) Labelled 6X-Tel and unlabelled B-6X-Tel or labelled 13X-Tel and unlabelled B-13X-Tel probes were incubated with 0, 0.2, 0.4 or 0.6 µM TRF1, followed by incubation with buffer (control), 0.25 µM TIN2 or 0.25 µM each of TIN2 or TIN2-13. The amount of probe released by phenol (measured as c.p.m.) was quantified by scintillation counting, and the relative change compared with 0.2 mM TRF1 is shown. (C) Stimulation induced by TIN2 or TIN2 and TIN2-13 was calculated by quantifying the radioactivity released in reactions containing both TIN2 and TRF1 and dividing this by the radioactivity released in reactions containing only TRF1 ((TRF1+TIN2) radioactivity/TRF1 radioactivity). ND, no data.
Similar articles
- TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex.
Ye JZ, de Lange T. Ye JZ, et al. Nat Genet. 2004 Jun;36(6):618-23. doi: 10.1038/ng1360. Epub 2004 May 9. Nat Genet. 2004. PMID: 15133513 - Structure, dynamics, and regulation of TRF1-TIN2-mediated trans- and cis-interactions on telomeric DNA.
Pan H, Kaur P, Barnes R, Detwiler AC, Sanford SL, Liu M, Xu P, Mahn C, Tang Q, Hao P, Bhattaram D, You C, Gu X, Lu W, Piehler J, Xu G, Weninger K, Riehn R, Opresko PL, Wang H. Pan H, et al. J Biol Chem. 2021 Sep;297(3):101080. doi: 10.1016/j.jbc.2021.101080. Epub 2021 Aug 14. J Biol Chem. 2021. PMID: 34403696 Free PMC article. - Human Telomere Repeat Binding Factor TRF1 Replaces TRF2 Bound to Shelterin Core Hub TIN2 when TPP1 Is Absent.
Janovič T, Stojaspal M, Veverka P, Horáková D, Hofr C. Janovič T, et al. J Mol Biol. 2019 Aug 9;431(17):3289-3301. doi: 10.1016/j.jmb.2019.05.038. Epub 2019 May 31. J Mol Biol. 2019. PMID: 31158366 - Regulation of telomerase by telomeric proteins.
Smogorzewska A, de Lange T. Smogorzewska A, et al. Annu Rev Biochem. 2004;73:177-208. doi: 10.1146/annurev.biochem.73.071403.160049. Annu Rev Biochem. 2004. PMID: 15189140 Review. - Telomere biology: a new player in the end zone.
Colgin L, Reddel R. Colgin L, et al. Curr Biol. 2004 Oct 26;14(20):R901-2. doi: 10.1016/j.cub.2004.09.075. Curr Biol. 2004. PMID: 15498484 Review.
Cited by
- Assembly path dependence of telomeric DNA compaction by TRF1, TIN2, and SA1.
Liu M, Pan H, Kaur P, Wang LJ, Jin M, Detwiler AC, Opresko PL, Tao YJ, Wang H, Riehn R. Liu M, et al. Biophys J. 2023 May 16;122(10):1822-1832. doi: 10.1016/j.bpj.2023.04.014. Epub 2023 Apr 20. Biophys J. 2023. PMID: 37081787 Free PMC article. - Non-canonical roles of canonical telomere binding proteins in cancers.
Akincilar SC, Chan CHT, Ng QF, Fidan K, Tergaonkar V. Akincilar SC, et al. Cell Mol Life Sci. 2021 May;78(9):4235-4257. doi: 10.1007/s00018-021-03783-0. Epub 2021 Feb 18. Cell Mol Life Sci. 2021. PMID: 33599797 Free PMC article. Review. - A Truncating Germline Mutation of TINF2 in Individuals with Thyroid Cancer or Melanoma Results in Longer Telomeres.
He H, Li W, Comiskey DF, Liyanarachchi S, Nieminen TT, Wang Y, DeLap KE, Brock P, de la Chapelle A. He H, et al. Thyroid. 2020 Feb;30(2):204-213. doi: 10.1089/thy.2019.0156. Thyroid. 2020. PMID: 31928178 Free PMC article. - The Role of Ubiquitination and SUMOylation in Telomere Biology.
Zalzman M, Meltzer WA, Portney BA, Brown RA, Gupta A. Zalzman M, et al. Curr Issues Mol Biol. 2020;35:85-98. doi: 10.21775/cimb.035.085. Epub 2019 Aug 18. Curr Issues Mol Biol. 2020. PMID: 31422934 Free PMC article. Review. - Molecular Architecture of Full-length TRF1 Favors Its Interaction with DNA.
Boskovic J, Martinez-Gago J, Mendez-Pertuz M, Buscato A, Martinez-Torrecuadrada JL, Blasco MA. Boskovic J, et al. J Biol Chem. 2016 Oct 7;291(41):21829-21835. doi: 10.1074/jbc.M116.744896. Epub 2016 Aug 25. J Biol Chem. 2016. PMID: 27563064 Free PMC article.
References
- Bilaud T., Brun C., Ancelin K., Koering C.E., Laroche T. & Gilson E. (1997) Telomeric localization of TRF2, a novel human telobox protein. Nature Genet., 17, 236–239. - PubMed
- Broccoli D., Smogorzewska A., Chong L. & de Lange T. (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nature Genet., 17, 231–235. - PubMed
- Campisi J., Kim S.H., Lim C.S. & Rubio M. (2001) Cellular senescence, cancer and aging: the telomere connection. Exp. Gerontol., 36, 1619–1637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials