Telomerase activity in B-cell non-Hodgkin lymphomas is regulated by hTERT transcription and correlated with telomere-binding protein expression but uncoupled from proliferation - PubMed (original) (raw)
Telomerase activity in B-cell non-Hodgkin lymphomas is regulated by hTERT transcription and correlated with telomere-binding protein expression but uncoupled from proliferation
W Klapper et al. Br J Cancer. 2003.
Abstract
Telomere maintenance is a prerequisite for immortalisation, and in most malignant cells is carried out by telomerase, an enzyme that synthesis new telomeric repeats on the chromosome ends. In normal or reactive tissues with a high regenerative capacity, telomerase is regulated according to the telomere loss that occurs during proliferation. To evaluate the interaction of proliferation and telomerase activity in malignant lymphomas, we quantified telomerase expression in different non-Hodgkin lymphomas in comparison to normal or reactive lymph nodes. Surprisingly, the activity levels were the same in most of the lymphomas analysed as compared to reactive lymph nodes. Significantly higher activity was detected only in Burkitt's lymphoma. Telomerase activity correlated well with hTERT and c-myc expression, but was independent of proliferation. To evaluate interactions of telomere-binding protein expression on telomerase expression in non-Hodgkin lymphoma, the mRNA levels of TRF1, TRF2, tankyrase and hPif1 were assessed by real-time RT-PCR. We demonstrate here that the magnitude of telomerase upregulation does not necessarily reflect the requirement of telomere compensation caused by proliferation. Telomerase regulation in non-Hodgkin lymphomas is therefore uncoupled from proliferative stimuli found in reactive lymphoid tissue. We suggest that the upregulation of specific telomere-binding proteins like TRF2 may contribute to telomere maintenance in malignant lymphoma.
Figures
Figure 1
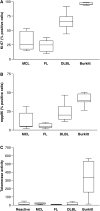
Proliferation measured by staining for the Ki67 (A) antigen and for repp86 (B). The percentage of positive cells was counted. In contrast to the repp86 antigen (detected by Ki-S2), the Ki-67 antigen is also expressed during G1-phase of the cell cycle. Thus, the values for Ki-67 are higher than for repp86 staining (one-way analysis of variance, P<0.001 for both antibodies). The proliferative index cannot be determined for benign lymph nodes reliably because proliferation is unevenly distributed throughought the tissue. (C) Telomerase activity measured by a semiquantitative TRAP. The activity is expressed as nanograms of an HL60 control cell line. In total, 200 ng experimental sample was used in each assay. No significant differences were found between benign lymph nodes (benign), MCL, FL and DLBL. High activity was measured in Burkitt's lymphoma (Burkitt) (one-way analysis of variance, P<0.001).
Figure 2
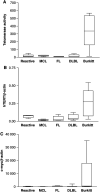
(A) Telomerase activity measured by a semiquantitative TRAP as shown in Figure 1C. (B) HTERT mRNA expression detected by real-time RT–PCR using a LightCycler Assay. The expression level of hTERT in the lymphomas is similar to telomerase activity (one-way analysis of variance: P<0.0018, correlation: _P_=0.0025, _r_=0.9826). (C) C-myc expression detected with real-time RT–PCR using a TaqMan assay. C-myc is strongly overexpressed in Burkitt's lymphoma, which carry the t(8;14) translocation (one-way analysis of variance: _P_=0.0079).
Figure 3
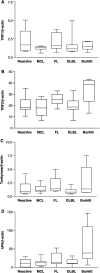
Telomere-binding protein expression detected by real-time RT–PCR using TaqMan assays. The expression is indicated relative to _β_-actin endogenous control: (A) TRF1 (one-way analysis of variance: _P_=0.433); (B) TRF2 (one-way analysis of variance: _P_=0.0239), (C) tankyrase (one-way analysis of variance: _P_=0.508), (D) hPif1 (one-way analysis of variance: _P_=0.0105).
Similar articles
- Expression of telomeric repeat binding factor 1 and 2 and TRF1-interacting nuclear protein 2 in human gastric carcinomas.
Matsutani N, Yokozaki H, Tahara E, Tahara H, Kuniyasu H, Haruma K, Chayama K, Yasui W, Tahara E. Matsutani N, et al. Int J Oncol. 2001 Sep;19(3):507-12. Int J Oncol. 2001. PMID: 11494028 - Elevated telomerase activity, c-MYC-, and hTERT mRNA expression: association with tumour progression in malignant lipomatous tumours.
Schneider-Stock R, Boltze C, Jäger V, Epplen J, Landt O, Peters B, Rys J, Roessner A. Schneider-Stock R, et al. J Pathol. 2003 Apr;199(4):517-25. doi: 10.1002/path.1315. J Pathol. 2003. PMID: 12635143 - hTERT expression in sporadic renal cell carcinomas.
Paradis V, Bièche I, Dargère D, Bonvoust F, Ferlicot S, Olivi M, Lagha NB, Blanchet P, Benoît G, Vidaud M, Bedossa P. Paradis V, et al. J Pathol. 2001 Sep;195(2):209-17. doi: 10.1002/path.917. J Pathol. 2001. PMID: 11592100 - Expression of telomerase genes in thyroid carcinoma.
Hoang-Vu C, Boltze C, Gimm O, Poremba C, Dockhorn-Dworniczak B, Köhrle J, Rath FW, Dralle H. Hoang-Vu C, et al. Int J Oncol. 2002 Aug;21(2):265-72. Int J Oncol. 2002. PMID: 12118320 Review. - [Telomerase, cell immortality and cancer].
Watanabe N. Watanabe N. Hokkaido Igaku Zasshi. 2001 May;76(3):127-32. Hokkaido Igaku Zasshi. 2001. PMID: 11481865 Review. Japanese.
Cited by
- The DNA methylation status of the TERT promoter differs between subtypes of mature B-cell lymphomas.
Kouroukli AG, Fischer A, Kretzmer H, Chteinberg E, Rajaram N, Glaser S, Kolarova J, Bashtrykov P, Mathas S, Drexler HG, Ohno H, Ammerpohl O, Jeltsch A, Siebert R, Bens S. Kouroukli AG, et al. Blood Cancer J. 2023 Jun 26;13(1):98. doi: 10.1038/s41408-023-00872-0. Blood Cancer J. 2023. PMID: 37365157 Free PMC article. No abstract available. - Targeting β-catenin using XAV939 nanoparticle promotes immunogenic cell death and suppresses conjunctival melanoma progression.
Antony F, Kang X, Pundkar C, Wang C, Mishra A, Chen P, Babu RJ, Suryawanshi A. Antony F, et al. Int J Pharm. 2023 Jun 10;640:123043. doi: 10.1016/j.ijpharm.2023.123043. Epub 2023 May 11. Int J Pharm. 2023. PMID: 37172631 Free PMC article. - Prognostic significance of dysregulation of shelterin complex and its correlation with telomere length and cytogenetics in multiple myeloma.
Kalal AA, Shetty RA, Manjappa AB, Kulkarni NV, Shetty P. Kalal AA, et al. J Genet Eng Biotechnol. 2023 May 3;21(1):50. doi: 10.1186/s43141-023-00504-x. J Genet Eng Biotechnol. 2023. PMID: 37131110 Free PMC article. - Biological Functions of TNKS1 and Its Relationship with Wnt/β-Catenin Pathway in Astrocytoma.
Chen M, Tang B, Xie S, Yan J, Yang L, Zhou X, Zeng E. Chen M, et al. Onco Targets Ther. 2019 Dec 11;12:10841-10850. doi: 10.2147/OTT.S206142. eCollection 2019. Onco Targets Ther. 2019. PMID: 31849489 Free PMC article. - Inhibitory effects of XAV939 on the proliferation of small-cell lung cancer H446 cells and Wnt/β-catenin signaling pathway in vitro.
Pan F, Shen F, Yang L, Zhang L, Guo W, Tian J. Pan F, et al. Oncol Lett. 2018 Aug;16(2):1953-1958. doi: 10.3892/ol.2018.8790. Epub 2018 May 24. Oncol Lett. 2018. PMID: 30008888 Free PMC article.
References
- Blackburn EH (1991) Structure and function of telomeres. Nature 350: 569–573 - PubMed
- Bonatz G, Klapper W, Barthe A, Heidorn K, Jonat W, Krupp G, Parwaresch R (1998) Analysis of telomerase expression and proliferative activity in the different layers of cyclic endometrium. Biochem Biophys Res Commun 253: 214–221 - PubMed
- Brousset P, al Saati T, Chaouche N, Zenou RC, Schlaifer D, Chittal S, Delsol G (1997) Telomerase activity in reactive and neoplastic lymphoid tissues: infrequent detection of activity in Hodgkin's disease. Blood 89: 26–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous