Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade - PubMed (original) (raw)
Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade
Isabelle Vergne et al. J Exp Med. 2003.
Abstract
The capacity of Mycobacterium tuberculosis to infect latently over one billion people and cause two million fatalities annually rests with its ability to block phagosomal maturation into the phagolysosome in infected macrophages. Here we describe how M. tuberculosis toxin lipoarabinomannan (LAM) causes phagosome maturation arrest, interfering with a new pathway connecting intracellular signaling and membrane trafficking. LAM from virulent M. tuberculosis, but not from avirulent mycobacteria, blocked cytosolic Ca2+ increase. Ca2+ and calmodulin were required for a newly uncovered Ca2+/calmodulin phosphatidylinositol (PI)3 kinase hVPS34 cascade, essential for production of PI 3 phosphate (PI3P) on liposomes in vitro and on phagosomes in vivo. The interference of the trafficking toxin LAM with the calmodulin-dependent production of PI3P described here ensures long-term M. tuberculosis residence in vacuoles sequestered away from the bactericidal and antigen-processing organelles in infected macrophages.
Figures
Figure 1.
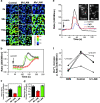
M. tuberculosis LAM inhibits [Ca2**+**]c rise. J774 cells were preincubated 30 min without or with 20 μg/ml M. tuberculosis LAM (Mt LAM) or M. smegmatis LAM (Ms LAM). (A) Ratio (340/380 nm) images at different time points after the addition of 1 μM A23187 untreated (control), Mt LAM–treated, or Ms LAM–treated J774 cells. (B) [Ca2+]c kinetics (mean ± SEM, n = 30 cells). Lines: black, untreated cells; red, Mt LAM-treated cells; green, Ms LAM–treated cells. (C) Rate of [Ca2+]c increase ± SEM (n = 3 independent experiments, mean of 30 cells per experiment). (D) Maximum initial [Ca2+]c increase ± SEM (n = 3 independent experiments, mean of 30 cells per experiment). (E) [Ca2+]c kinetics after FcR cross-linking (refer to Materials and Methods) on J774 cells treated with 10 μM DMS (blue line), 20 μg/ml Mt LAM (red line), or control (black line). Mean of 65 cells (same field). FcR clustering revealed by Texas Red anti–mouse F(ab')2 fragment in presence (E”) or absence (E') of Mt LAM. (F) [Ca2+]c increase upon FcR cross-linking in the absence or presence of 10 μM DMS or 20 μg/ml Mt. LAM. Four independent experiments, mean of 65 cells per experiment, are shown. P-values, paired t test (
).
Figure 2.
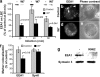
Ca2+/calmodulin and CaMKII positively regulate EEA1 recruitment to phagosomes. (A) Immunoblotting analysis and quantitation (mean ± SE) of EEA1 on purified LBC isolated from 25 μM W7-treated or -untreated macrophages. (B–E) Immunofluorescence images of EEA1 recruited to phagosomes (B and D) and corresponding phase contrast images (C and E) in W7-treated (D and E) or -untreated (B and C) macrophages. Triangles indicate EEA1 colocalization with phagosomes. (F) Phagosome colocalization quantitation of EEA1 and Syntaxin 8 by immunofluorescence (mean ± SE; n = 1,145 phagosomes, 35 fields). (G) Immunoblotting analysis of EEA1 and Syntaxin 3 on purified phagosomes from 2 μM KN62-treated and -untreated cells.
Figure 3.
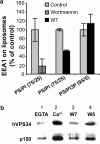
Ca2+/calmodulin and PI3 kinase–dependent association of EEA1 with liposomes and Ca2+-dependent binding of calmodulin to a protein complex containing PI3 kinase hVPS34. (A) Quantitation (mean ± SE) of EEA1 on phosphatidylserine (PS) and phosphatidylinositol (PI) or PS and PI3P liposomes after incubation with J774 cytosol in the presence or absence of 100 nM PI3 kinase inhibitor wortmannin or 100 μM calmodulin inhibitor W7. (B) Immunoblotting analysis of hVPS34 and p150 on calmodulin agarose beads after incubation with J774 cytosol in the presence of EGTA (lane 1), Ca2+ (lanes 2, 3, and 4), W7 (lane 3), and W5 (lane 4).
Figure 4.
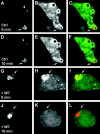
Calmodulin positively regulates PI3P on phagosomes. RAW 264.7 macrophages, transfected with the PI3P probe p40PX-EGFP, were imaged live while phagocytosing latex beads using an UltraView confocal microscope. After latex bead internalization and determination of PI3P positivity of the LBC, solvent without (Ctrl) or with 25 μM W7 (+W7) were added to the chamber. Panels: a, d, g, and j, Texas Red–labeled latex beads; b, e, h, and k, GFP probe for PI3P; c, f, i, and l, merged red and green channel images. Note localization of PI3P GFP probe on LBC after bead internalization (a–c and g–i), continued presence of the PI3P GFP probe on phagosomes 10 min after the addition of solvent alone (d–f), and the disappearance of the PI3P GFP probe when W7 was added (j–l). Arrows depict representative LBC in each experimental set. Examples of three independent experiments with comparable results are shown. Also, see Video 1 (available at
http://www.jem.org/cgi/content/full/jem.20030527/DC1
), which corresponds to panels g–l.
Similar articles
- Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest.
Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Fratti RA, et al. J Cell Biol. 2001 Aug 6;154(3):631-44. doi: 10.1083/jcb.200106049. J Cell Biol. 2001. PMID: 11489920 Free PMC article. - Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis.
Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Vergne I, et al. Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):4033-8. doi: 10.1073/pnas.0409716102. Epub 2005 Mar 7. Proc Natl Acad Sci U S A. 2005. PMID: 15753315 Free PMC article. - Mycobacterium tuberculosis phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking.
Vergne I, Chua J, Deretic V. Vergne I, et al. Traffic. 2003 Sep;4(9):600-6. doi: 10.1034/j.1600-0854.2003.00120.x. Traffic. 2003. PMID: 12911814 Review. - Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles.
Chua J, Deretic V. Chua J, et al. J Biol Chem. 2004 Aug 27;279(35):36982-92. doi: 10.1074/jbc.M405082200. Epub 2004 Jun 21. J Biol Chem. 2004. PMID: 15210698 - Cell biology of mycobacterium tuberculosis phagosome.
Vergne I, Chua J, Singh SB, Deretic V. Vergne I, et al. Annu Rev Cell Dev Biol. 2004;20:367-94. doi: 10.1146/annurev.cellbio.20.010403.114015. Annu Rev Cell Dev Biol. 2004. PMID: 15473845 Review.
Cited by
- Macrophages in tuberculosis: friend or foe.
Guirado E, Schlesinger LS, Kaplan G. Guirado E, et al. Semin Immunopathol. 2013 Sep;35(5):563-83. doi: 10.1007/s00281-013-0388-2. Epub 2013 Jul 18. Semin Immunopathol. 2013. PMID: 23864058 Free PMC article. Review. - Ion channels in innate and adaptive immunity.
Feske S, Wulff H, Skolnik EY. Feske S, et al. Annu Rev Immunol. 2015;33:291-353. doi: 10.1146/annurev-immunol-032414-112212. Annu Rev Immunol. 2015. PMID: 25861976 Free PMC article. Review. - Calmodulin (CaM) Activates PI3Kα by Targeting the "Soft" CaM-Binding Motifs in Both the nSH2 and cSH2 Domains of p85α.
Zhang M, Li Z, Wang G, Jang H, Sacks DB, Zhang J, Gaponenko V, Nussinov R. Zhang M, et al. J Phys Chem B. 2018 Dec 13;122(49):11137-11146. doi: 10.1021/acs.jpcb.8b05982. Epub 2018 Aug 8. J Phys Chem B. 2018. PMID: 30047727 Free PMC article. - Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2alpha on neurosecretory vesicles.
Wen PJ, Osborne SL, Morrow IC, Parton RG, Domin J, Meunier FA. Wen PJ, et al. Mol Biol Cell. 2008 Dec;19(12):5593-603. doi: 10.1091/mbc.e08-06-0595. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843041 Free PMC article. - Malnutrition: Modulator of Immune Responses in Tuberculosis.
Chandrasekaran P, Saravanan N, Bethunaickan R, Tripathy S. Chandrasekaran P, et al. Front Immunol. 2017 Oct 18;8:1316. doi: 10.3389/fimmu.2017.01316. eCollection 2017. Front Immunol. 2017. PMID: 29093710 Free PMC article. Review.
References
- Meresse, S., O. Steele-Mortimer, E. Moreno, M. Desjardins, B. Finlay, and J.P. Gorvel. 1999. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1:E183–E188. - PubMed
- Christoforidis, S., H.M. McBride, R.D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 397:621–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous