Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity - PubMed (original) (raw)
Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity
Patrick C Reading et al. J Virol. 2003 Sep.
Abstract
Interleukin-18 (IL-18) is a proinflammatory cytokine that promotes natural killer (NK) and T-cell activation. Several poxviruses, including vaccinia virus (VV), encode a soluble IL-18-binding protein (IL-18bp). The role of the VV IL-18bp (gene C12L) in vivo was studied with wild-type (vC12L), deletion mutant (vDeltaC12L), and revertant (vC12L-rev) viruses in a murine intranasal model of infection. The data show that vDeltaC12L was markedly attenuated, characterized by a mild weight loss and reduced virus titers in lungs, brain, and spleen. Three days after infection, NK cytotoxic activity was augmented in the lung, spleen, and mediastinal lymph nodes (MLNs) of vDeltaC12L-infected mice compared to controls. Seven days after infection, vDeltaC12L-infected mice displayed heightened VV-specific cytotoxic T-lymphocyte (CTL) responses in the lungs, spleen, and MLNs. Gamma interferon (IFN-gamma) levels were also dramatically elevated in lavage fluids and cells from lungs of mice infected with vDeltaC12L. Finally, we demonstrate that IL-18 is produced in vitro and in vivo after VV infection. Taken together, these data demonstrate a role for the vIL-18bp in counteracting IL-18 in both the innate and the specific immune response to VV infection and indicate that the ability of IL-18 to promote vigorous T-cell responses (cytotoxic activity and IFN-gamma production) is a critical factor in the accelerated clearance of the vDeltaC12L mutant.
Figures
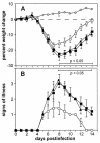
FIG. 1.
Virulence of recombinant VVs in the murine intranasal model. Groups of five BALB/c mice were mock-infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). (A) Mice were weighed daily, and the results are expressed as the mean percent weight loss of each group ± standard error compared with the weight immediately prior to infection. Data are from five mice, except for the group infected with vC12L-rev, in which one mouse was sacrificed at day 9 and another at day 10. P values were determined by using Student's t test and indicate mean percent weight changes of mice infected with vΔC12L that were significantly different from those of mice infected with vC12L or vC12L-rev. (B) Animals were monitored daily for signs of illness, which was scored from 1 to 4 as described previously (2). Data from each day are expressed as the mean ± standard error from five mice, except for the group infected with C12L-rev, in which 1 mouse was sacrificed at day 9 and another at day 10. P values were determined by using Student's t test and indicate mean signs of illness of mice infected with vΔC12L that were significantly different from those of mice infected with vC12L or vC12L-rev.
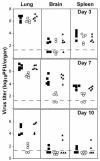
FIG. 2.
Replication and spread of recombinant VVs in vivo. Mice were infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). On the indicated day p.i., five animals infected with each virus were sacrificed, and the titer of infectious virus in the lungs, brains, and spleens was determined by plaque assay on BS-C-1 cells. Virus titers are expressed as PFU per organ. The broken line indicates the minimum detection limit of the plaque assay.
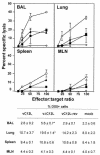
FIG. 3.
NK cytotoxicity in BAL, lung, spleen, and MLN after VV infection. Groups of six mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At day 3, NK cytotoxicity was determined in cell suspensions prepared from the BAL, lung, spleen, and MLN of mice. Specific lysis of YAC-1 cells was assessed by 51Cr-release assay. Data are expressed as the mean ± standard error from two groups of mice (n = 3 per group). Cells were also stained for expression of the pan-NK cell marker DX5 and examined by flow cytometry with a minimum of 20,000 cells analyzed in a lymphocyte gate. Results are expressed as the percentage of DX5+ cells in the total viable cell population. P values were determined by Student's t test and indicate the mean percentage of DX5+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05.
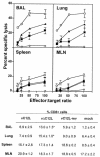
FIG. 4.
CTL activity in BAL, lung, spleen, and MLN after VV infection. Groups of six mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At day 7, CTL activity was determined in cell suspensions prepared from BAL, lung, spleen, and MLN of mice. Specific lysis of WR-infected P815 cells was assessed by 51Cr-release assay. Data are expressed as the mean ± standard error from two groups of mice (n = 3 per group). Lysis of noninfected P815s by day 7 effector cell populations was always <10% at an E:T ratio of 100:1 and is not shown. Cells were also stained for expression of CD8 and examined by flow cytometry with a minimum of 20,000 cells analyzed in a lymphocyte gate. Results are expressed as the percentage of CD8+ cells in the total viable cell population. P values were determined by Student's t test and indicate the mean percentage of CD8+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05.
FIG. 5.
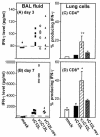
Production of IFN-γ in the lungs of VV-infected mice. Groups of four to six BALB/c mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At days 3 and 7, mice were sacrificed, BALs were performed, and single-cell suspensions were prepared from lung tissue. (A and B) Levels of IFN-γ in BAL fluids of VV-infected mice. BAL samples were centrifuged, and the levels of IFN-γ present in the supernatant were determined by ELISA at days 3 and 7. Values for individual mice are shown. The broken line represents the minimum detection level of the assay. (C and D) Intracellular production of IFN-γ by lung lymphocytes from mice 7 days pi. Lung cells were stimulated with PMA and iomnomycin for 4 h, and brefeldin A was added to retain cytokines in the cytoplasm. Cells were stained with FITC-labeled anti-CD8, QR-labeled anti-CD4, and, after permeabilization with saponin, PE-labeled anti-IFN-γ before analysis by three-color flow cytometry. Shown is the percentage of CD4+ (C) or CD8+ (D) T lymphocytes producing IFN-γ. Values are averaged from two groups (n = 3 per group). P values were determined by Student's t test and indicate the mean percentage of CD8+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05; **, P < 0.02.
FIG. 6.
Nitrite levels in the cell-free BAL of mock-infected mice or mice infected with 104 PFU of vC12L, vΔC12L, or vC12L-rev. The data for each time point represent the mean nitrite level ± standard error of four to five individual mice. Columns marked with an asterisk indicate nitrite levels in BAL fluid from vΔC12L-infected mice that were statistically different from those from both vC12L- and vC12L-rev-infected animals. **, P < 0.02.
FIG. 7.
IL-18 production in response to VV infection in vivo and in vitro. (A) IL-18 levels in BAL. Mice were infected with 104 PFU of vC12L, vΔC12L, or vC12L-rev and were sacrificed 3 or 7 days later. BALs from four to five mice were pooled, and the level of total IL-18 protein was determined by ELISA. Data are expressed as the mean ± standard error from two independent experiments. (B) IL-18 release by lung macrophages. Lung macrophages were isolated from naive BALB/c mice and cultured in the presence of 10 PFU of vC12L, vΔC12L, or C12L-rev per cell. Supernatants were measured for IL-18 by ELISA. Results are expressed as the mean ± standard error of duplicate wells.
Similar articles
- The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virus virulence in the murine intranasal model.
Symons JA, Adams E, Tscharke DC, Reading PC, Waldmann H, Smith GL. Symons JA, et al. J Gen Virol. 2002 Nov;83(Pt 11):2833-2844. doi: 10.1099/0022-1317-83-11-2833. J Gen Virol. 2002. PMID: 12388820 - Steroid hormone synthesis by vaccinia virus suppresses the inflammatory response to infection.
Reading PC, Moore JB, Smith GL. Reading PC, et al. J Exp Med. 2003 May 19;197(10):1269-78. doi: 10.1084/jem.20022201. J Exp Med. 2003. PMID: 12756265 Free PMC article. - Interleukin 17 modulates the immune response to vaccinia virus infection.
Patera AC, Pesnicak L, Bertin J, Cohen JI. Patera AC, et al. Virology. 2002 Jul 20;299(1):56-63. doi: 10.1006/viro.2002.1400. Virology. 2002. PMID: 12167341 - Alpha beta and gamma delta T-cell networks and their roles in natural resistance to viral infections.
Welsh RM, Lin MY, Lohman BL, Varga SM, Zarozinski CC, Selin LK. Welsh RM, et al. Immunol Rev. 1997 Oct;159:79-93. doi: 10.1111/j.1600-065x.1997.tb01008.x. Immunol Rev. 1997. PMID: 9416504 Review. - Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP.
Fabbi M, Carbotti G, Ferrini S. Fabbi M, et al. J Leukoc Biol. 2015 Apr;97(4):665-75. doi: 10.1189/jlb.5RU0714-360RR. Epub 2014 Dec 29. J Leukoc Biol. 2015. PMID: 25548255 Review.
Cited by
- Genetic variation in IL18R1 and IL18 genes and Inteferon γ ELISPOT response to smallpox vaccination: an unexpected relationship.
Ovsyannikova IG, Haralambieva IH, Kennedy RB, O'Byrne MM, Pankratz VS, Poland GA. Ovsyannikova IG, et al. J Infect Dis. 2013 Nov 1;208(9):1422-30. doi: 10.1093/infdis/jit341. Epub 2013 Jul 30. J Infect Dis. 2013. PMID: 23901078 Free PMC article. - Natural killer cells in immunodefense against infective agents.
Zucchini N, Crozat K, Baranek T, Robbins SH, Altfeld M, Dalod M. Zucchini N, et al. Expert Rev Anti Infect Ther. 2008 Dec;6(6):867-85. doi: 10.1586/14787210.6.6.867. Expert Rev Anti Infect Ther. 2008. PMID: 19053900 Free PMC article. Review. - Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses.
Earl PL, Americo JL, Moss B. Earl PL, et al. PLoS Pathog. 2020 Apr 22;16(4):e1008505. doi: 10.1371/journal.ppat.1008505. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32320436 Free PMC article. - Vaccinia virus vaccines: past, present and future.
Jacobs BL, Langland JO, Kibler KV, Denzler KL, White SD, Holechek SA, Wong S, Huynh T, Baskin CR. Jacobs BL, et al. Antiviral Res. 2009 Oct;84(1):1-13. doi: 10.1016/j.antiviral.2009.06.006. Epub 2009 Jun 26. Antiviral Res. 2009. PMID: 19563829 Free PMC article. Review. - The poxvirus A35 protein is an immunoregulator.
Rehm KE, Jones GJ, Tripp AA, Metcalf MW, Roper RL. Rehm KE, et al. J Virol. 2010 Jan;84(1):418-25. doi: 10.1128/JVI.01802-09. J Virol. 2010. PMID: 19828608 Free PMC article.
References
- Alcamí, A., and G. L. Smith. 1992. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71:153-167. - PubMed
- Alcamí, A., and G. L. Smith. 2002. The vaccinia virus soluble interferon-gamma receptor is a homodimer. J. Gen. Virol. 83:545-549. - PubMed
- Alcamí, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160:624-633. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous