An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1 - PubMed (original) (raw)
An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1
Jana Gerber et al. EMBO Rep. 2003 Sep.
Abstract
Depletion of the mitochondrial matrix protein frataxin is the molecular cause of the neurodegenerative disease Friedreich ataxia. The function of frataxin is unclear, although recent studies have suggested a function of frataxin (yeast Yfh1) in iron/sulphur (Fe/S) protein biogenesis. Here, we show that Yfh1 specifically binds to the central Fe/S-cluster (ISC)-assembly complex, which is composed of the scaffold protein Isu1 and the cysteine desulphurase Nfs1. Association between Yfh1 and Isu1/Nfs1 was markedly increased by ferrous iron, but did not depend on ISCs on Isu1. Functional analyses in vivo showed an involvement of Yfh1 in de novo ISC synthesis on Isu1. Our data demonstrate a crucial function of Yfh1 in Fe/S protein biogenesis by defining its function in an early step of this essential process. The iron-dependent binding of Yfh1 to Isu1/Nfs1 suggests a role of frataxin/Yfh1 in iron loading of the Isu scaffold proteins.
Figures
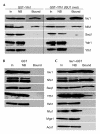
Figure 1
Yfh1 binds to the Isu1/Nfs1 complex. The fusion proteins pSu9GST–Yfh1, pSu9GST and Isu1–GST (glutathione-S_-transferase) were produced in wild-type yeast cells grown in the presence of galactose. Mitochondria were isolated, lysed in detergent-containing buffer A, and GST-affinity purification was performed. Fractions were analysed for the presence of specific proteins by immunostaining (A–C). Results obtained using cells that overexpressed_ISU1 are shown in the right panel of (A) (ISU1 over.). Bound, GST-affinity purified protein; In, input mitochondrial extract (10% of total); NB, non-bound fraction (10% of total).
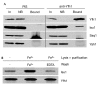
Figure 2
The interaction between Isu1–GST and Yfh1 is dependent on the concentrations of Isu1 and Yfh1. (A) Wild-type (WT) and_Gal–ISU1/Δisu2_ cells were grown on synthetic minimal media supplemented with galactose (Gal) or glucose (D). Mitochondria were isolated and analysed by immunostaining for Isu1, porin (Por2) and Mge1. (B,C) Isu1–GST was overproduced in Gal–ISU1/Δisu2 cells containing a plasmid (pCM182–YFH1) that carries YFH1 under the control of the TetO2 promoter. In this strain, levels of native Isu1 (without GST) are downregulated by growth in the presence of glucose, and the overexpression of YFH1 is blocked by the addition of 5 μg ml−1 doxycycline. Mitochondria were isolated from cells cultivated on synthetic minimal media supplemented with galactose (ISU1 up) or glucose (ISU1 down) in the absence (YFH1 up) or presence (YFH1 down) of doxycycline, as indicated. Further analysis was carried out as described for Fig. 1. Bound, glutathione-_S_-transferase (GST)-affinity purified protein; In, input mitochondrial extract (10% of total); NB, non-bound fraction (10% of total).
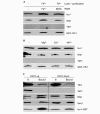
Figure 3
Co-immunoprecipitation of Yfh1 and the Isu1/Nfs1 complex. (A) Isolated wild-type mitochondria that overexpressed Isu1 (p426–Isu1) were lysed in detergent-containing buffer A and an immunoprecipitation was performed using a specific antiserum raised against purified Yfh1 and a pre-immune serum (PIS). The immunoprecipitates were analysed by SDS-polyacrylamide gel electrophoresis, followed by immunostaining for the indicated proteins. The staining below Ssq1 is due to the heavy chains of IgG, which preclude the analysis of Nfs1 by this method. (B) The lysis, purification and washing steps were carried out in the absence or presence of 50 μM Fe2+/1 mM ascorbate (Fe2+) and 1 mM EDTA, as indicated. Bound, glutathione-_S_-transferae (GST)-affinity purified protein; In, input mitochondrial extract (10% of total); NB, non-bound fraction (10% of total).
Figure 4
Binding of Isu1 to Yfh1 is increased at higher iron concentrations, but does not require an Fe/S cluster on Isu1. (A,B) Mitochondria from wild-type cells that overproduce glutathione-S-transferease (GST)–Yfh1 were used for GST-affinity purification, as described for Fig. 1. Treatment of samples in (A) was as described for Fig. 3B; samples in (B) were incubated in the presence of 50 μM of metal ions, as indicated. (C)Gal–YAH1 cells that overexpress Isu1–GST were grown on synthetic minimal media supplemented with galactose (YAH up) or glucose (YAH down). Mitochondria were isolated, Isu1–GST was purified and bound proteins were analysed as described for Fig. 1. Bound, GST-affinity purified protein; In, input mitochondrial extract (10% of total).
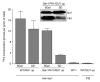
Figure 5
Yfh1 is required for de novo Fe/S-cluster synthesis on Isu1. Wild-type (WT) and Gal–YFH1 cells that overexpress ISU1 (ISU1 up) were incubated in an iron-poor medium supplemented with galactose (SGal) or glucose (SD). Cells were radiolabelled with55Fe and lysed mechanically, and Isu1 was immunoprecipitated from the cell lysates using Isu1-specific antibodies. The amount of radioactive55Fe associated with the immunobeads was quantified by liquid scintillation counting. The inset (immunostaining) shows the levels of Isu1 and Yfh1 in Gal–YFH1 cells. PIS, immunoprecipitation with pre-immune serum.
Similar articles
- Architecture of the Yeast Mitochondrial Iron-Sulfur Cluster Assembly Machinery: THE SUB-COMPLEX FORMED BY THE IRON DONOR, Yfh1 PROTEIN, AND THE SCAFFOLD, Isu1 PROTEIN.
Ranatunga W, Gakh O, Galeano BK, Smith DY 4th, Söderberg CA, Al-Karadaghi S, Thompson JR, Isaya G. Ranatunga W, et al. J Biol Chem. 2016 May 6;291(19):10378-98. doi: 10.1074/jbc.M115.712414. Epub 2016 Mar 3. J Biol Chem. 2016. PMID: 26941001 Free PMC article. - Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly.
Pandey A, Gordon DM, Pain J, Stemmler TL, Dancis A, Pain D. Pandey A, et al. J Biol Chem. 2013 Dec 27;288(52):36773-86. doi: 10.1074/jbc.M113.525857. Epub 2013 Nov 11. J Biol Chem. 2013. PMID: 24217246 Free PMC article. - Zinc and the iron donor frataxin regulate oligomerization of the scaffold protein to form new Fe-S cluster assembly centers.
Galeano BK, Ranatunga W, Gakh O, Smith DY, Thompson JR, Isaya G. Galeano BK, et al. Metallomics. 2017 Jun 21;9(6):773-801. doi: 10.1039/c7mt00089h. Metallomics. 2017. PMID: 28548666 Free PMC article. - Frataxin and mitochondrial FeS cluster biogenesis.
Stemmler TL, Lesuisse E, Pain D, Dancis A. Stemmler TL, et al. J Biol Chem. 2010 Aug 27;285(35):26737-26743. doi: 10.1074/jbc.R110.118679. Epub 2010 Jun 3. J Biol Chem. 2010. PMID: 20522547 Free PMC article. Review. - The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism.
Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Mühlenhoff U. Lill R, et al. Biochim Biophys Acta. 2012 Sep;1823(9):1491-508. doi: 10.1016/j.bbamcr.2012.05.009. Epub 2012 May 15. Biochim Biophys Acta. 2012. PMID: 22609301 Review.
Cited by
- The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation.
Uzarska MA, Dutkiewicz R, Freibert SA, Lill R, Mühlenhoff U. Uzarska MA, et al. Mol Biol Cell. 2013 Jun;24(12):1830-41. doi: 10.1091/mbc.E12-09-0644. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615440 Free PMC article. - Human frataxin, the Friedreich ataxia deficient protein, interacts with mitochondrial respiratory chain.
Doni D, Cavion F, Bortolus M, Baschiera E, Muccioli S, Tombesi G, d'Ettorre F, Ottaviani D, Marchesan E, Leanza L, Greggio E, Ziviani E, Russo A, Bellin M, Sartori G, Carbonera D, Salviati L, Costantini P. Doni D, et al. Cell Death Dis. 2023 Dec 8;14(12):805. doi: 10.1038/s41419-023-06320-y. Cell Death Dis. 2023. PMID: 38062036 Free PMC article. - Friedreich ataxia: new pathways.
Pandolfo M. Pandolfo M. J Child Neurol. 2012 Sep;27(9):1204-11. doi: 10.1177/0883073812448534. Epub 2012 Jun 29. J Child Neurol. 2012. PMID: 22752488 Free PMC article. - The power of yeast to model diseases of the powerhouse of the cell.
Baile MG, Claypool SM. Baile MG, et al. Front Biosci (Landmark Ed). 2013 Jan 1;18(1):241-78. doi: 10.2741/4098. Front Biosci (Landmark Ed). 2013. PMID: 23276920 Free PMC article. Review. - Gene expression profiling in frataxin deficient mice: microarray evidence for significant expression changes without detectable neurodegeneration.
Coppola G, Choi SH, Santos MM, Miranda CJ, Tentler D, Wexler EM, Pandolfo M, Geschwind DH. Coppola G, et al. Neurobiol Dis. 2006 May;22(2):302-11. doi: 10.1016/j.nbd.2005.11.014. Epub 2006 Jan 25. Neurobiol Dis. 2006. PMID: 16442805 Free PMC article.
References
- Adinolfi S., Trifuoggi M., Politou A.S., Martin S. & Pastore A. ( 2002) A structural approach to understanding the iron-binding properties of phylogenetically different frataxins. Hum. Mol. Genet., 11, 1865–1877. - PubMed
- Agar J.N., Yuvaniyama P., Jack R.F., Cash V.L., Smith A.D., Dean D.R. & Johnson M.K. ( 2000) Modular organization and identification of a mononuclear iron-binding site within the NifU protein. J. Biol. Inorg. Chem., 5, 167–177. - PubMed
- Babcock M., De Silva D., Oaks R., Davis-Kaplan S., Jiralerspong S., Montermini L., Pandolfo M. & Kaplan J. ( 1997) Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science, 276, 1709–1712. - PubMed
- Cavadini P., O'Neill H.A., Benada O. & Isaya G. ( 2002) Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum. Mol. Genet., 11, 217–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous