Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts - PubMed (original) (raw)
Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts
L E M Willemsen et al. Gut. 2003 Oct.
Abstract
Background: The mucus layer protects the gastrointestinal mucosa from mechanical, chemical, and microbial challenge. Mucin 2 (MUC-2) is the most prominent mucin secreted by intestinal epithelial cells. There is accumulating evidence that subepithelial myofibroblasts regulate intestinal epithelial cell function and are an important source of prostaglandins (PG). PG enhance mucin secretion and are key players in mucoprotection. The role of bacterial fermentation products in these processes deserves further attention.
Aims: We therefore determined whether the effect of short chain fatty acids (SCFA) on MUC-2 expression involves intermediate PG production.
Methods: Both mono- and cocultures of epithelial cells and myofibroblasts were used to study the effects of SCFA on MUC-2 expression and PG synthesis. Cell culture supernatants were used to determine the role of myofibroblast derived prostaglandins in increasing MUC-2 expression in epithelial cells.
Results: Prostaglandin E(1) (PGE(1)) was found to be far more potent than PGE(2) in stimulating MUC-2 expression. SCFA supported a mucoprotective PG profile, reflected by an increased PGE(1)/PGE(2) ratio in myofibroblast supernatants and increased MUC-2 expression in mono- and cocultures. Incubation with indomethacin revealed the latter to be mediated by PG.
Conclusions: SCFA can differentially regulate PG production, thus stimulating MUC-2 expression in intestinal epithelial cells. This mechanism involving functional interaction between myofibroblasts and epithelial cells may play an important role in the mucoprotective effect of bacterial fermentation products.
Figures
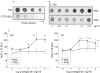
Figure 1
Prostaglandin stimulation of mucin 2 (MUC-2) expression in monocultures (MC)/cocultures (CC) of T84. (A) Dot blot analyses showed MC T84 cells to be positive for MUC-2 whereas CCD-18Co were negative. (B) Representative dot blot showing the effects of prostaglandins PGE1 and PGE2 on MUC-2 expression in CC T84. (C) Densitometric analysis revealed PGE1 to enhance MUC-2 expression in both MC and CC T84 (1–100 ng/ml; **p<0.002) whereas PGE2 only marginally affected MUC-2 expression in CC T84 (10–100 ng/ml; *p<0.01). MUC-2 expression is presented relative to controls (%BLU).
Figure 2
Short chain fatty acids (SCFA) increased the prostaglandin PGE1/PGE2 ratio produced by CCD-18Co. (A) Butyrate increased PGE1 and decreased PGE2 concentrations (*p<0.01, **p<0.002); acetate and propionate followed the same tendency. Prostaglandin production is presented relative to controls (% control). (B) SCFA increased the PGE1/PGE2 ratio (n = 5; *p<0.01, **p<0.002), resulting in a preferred mucoprotective profile.
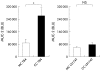
Figure 3
Increased mucin 2 (MUC-2) expression in cocultures (CC) of T84 compared with monocultures (MC) of T84. Basal MUC-2 expression increased when T84 cells were cocultured with CCD-18Co, the latter however was not apparent using LS174T cells (n = 4, *p<0.05). BLU, light units.
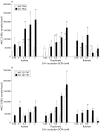
Figure 4
Short chain fatty acids (SCFA) increased mucin 2 (MUC-2) expression in monocultures (MC) and cocultures (CC) of T84 and LS174T. (A, B) SCFA stimulation of MUC-2 expression in the presence of CCD-18Co was found to be more pronounced compared with epithelial monolayers alone (p<0.05). Acetate and propionate dose dependently increased MUC-2 expression in MC/CC T84 (n = 4) and MC/CC LS174T (n = 3) (*p<0.01, **p<0.002). Butyrate was effective in MC/CC T84 and CC LS174T (p<0.01, p<0.002) but did not enhance MUC-2 expression in MC LS174T cells. MUC-2 expression was presented after deduction of basal expression levels of either MC or CC. BLU, light units.
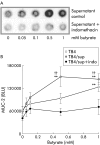
Figure 5
Mucin 2 (MUC-2) stimulation by butyrate is mediated by prostaglandins. (A) Representative dot blot of MUC-2 expression in monocultures of T84 incubated with supernatants of CCD-18Co that had been stimulated with butyrate in the presence or absence of indomethacin. (B) Densitometric analysis of four different experiments. Stimulation of MUC-2 expression by butyrate was found to be mediated by CCD-18Co and T84 derived prostaglandins as indomethacin (indo) blocked this effect (0.5-1 mM; ††p<0.01). CCD-18Co supernatants (sup) increased MUC-2 expression at lower butyrate concentrations (0.5–1 mM; **p<0.002) compared with MC T84 incubated with butyrate (1 mM; **p<0.002).
Similar articles
- Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose.
Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Gaudier E, et al. Am J Physiol Gastrointest Liver Physiol. 2004 Dec;287(6):G1168-74. doi: 10.1152/ajpgi.00219.2004. Epub 2004 Aug 12. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15308471 - Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis.
Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Shao J, et al. Cancer Res. 2006 Jan 15;66(2):846-55. doi: 10.1158/0008-5472.CAN-05-2606. Cancer Res. 2006. PMID: 16424017 - Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro.
Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Mack DR, et al. Gut. 2003 Jun;52(6):827-33. doi: 10.1136/gut.52.6.827. Gut. 2003. PMID: 12740338 Free PMC article. - [Gastrointestinal protective barrier].
Mogil'naia GM, Mogil'naia VL. Mogil'naia GM, et al. Morfologiia. 2007;132(6):9-16. Morfologiia. 2007. PMID: 18411716 Review. Russian. - [Human gastrointestinal tract mucins encoded by the MUC gene family].
Paszkiewicz-Gadek A, Porowska H. Paszkiewicz-Gadek A, et al. Postepy Hig Med Dosw. 2000;54(2):183-98. Postepy Hig Med Dosw. 2000. PMID: 10857379 Review. Polish.
Cited by
- The gut microbiome in cardio-metabolic health.
Hansen TH, Gøbel RJ, Hansen T, Pedersen O. Hansen TH, et al. Genome Med. 2015 Mar 31;7(1):33. doi: 10.1186/s13073-015-0157-z. eCollection 2015. Genome Med. 2015. PMID: 25825594 Free PMC article. Review. - Role of Gut Microbial Metabolites in the Pathogenesis of Primary Liver Cancers.
Pallozzi M, De Gaetano V, Di Tommaso N, Cerrito L, Santopaolo F, Stella L, Gasbarrini A, Ponziani FR. Pallozzi M, et al. Nutrients. 2024 Jul 22;16(14):2372. doi: 10.3390/nu16142372. Nutrients. 2024. PMID: 39064815 Free PMC article. Review. - Reevaluating the hype: four bacterial metabolites under scrutiny.
Fröhlich EE, Mayerhofer R, Holzer P. Fröhlich EE, et al. Eur J Microbiol Immunol (Bp). 2015 Mar;5(1):1-13. doi: 10.1556/EUJMI-D-14-00030. Epub 2015 Mar 26. Eur J Microbiol Immunol (Bp). 2015. PMID: 25883790 Free PMC article. Review. - Airway microbiome-immune crosstalk in chronic obstructive pulmonary disease.
Kayongo A, Robertson NM, Siddharthan T, Ntayi ML, Ndawula JC, Sande OJ, Bagaya BS, Kirenga B, Mayanja-Kizza H, Joloba ML, Forslund SK. Kayongo A, et al. Front Immunol. 2023 Jan 17;13:1085551. doi: 10.3389/fimmu.2022.1085551. eCollection 2022. Front Immunol. 2023. PMID: 36741369 Free PMC article. Review. - Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far?
Fukui H. Fukui H. Diseases. 2019 Nov 12;7(4):58. doi: 10.3390/diseases7040058. Diseases. 2019. PMID: 31726747 Free PMC article. Review.
References
- Casellas F, Papo M, Guarner F, et al. Intracolonic release in vivo of interleukin-1 beta in chronic ulcerative colitis. Clin Sci Colch 1995;89:521–6. - PubMed
- McKay DM, Croitoru K, Perdue MH. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol 1996;270:C418–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous