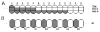Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila - PubMed (original) (raw)
Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila
D Moazed et al. Development. 1992 Nov.
Abstract
The stable maintenance of expression patterns of homeotic genes depends on the function of a number of negative trans-regulators, termed the Polycomb (Pc) group of genes. We have examined the pattern of expression of the Drosophila segment polarity gene, engrailed (en), in embryos mutant for several different members of the Pc group. Here we report that embryos mutant for two or more Pc group genes show strong ectopic en expression, while only weak derepression of en occurs in embryos mutant for a single Pc group gene. This derepression is independent of two known activators of en expression: en itself and wingless. Additionally, in contrast to the strong ectopic expression of homeotic genes observed in extra sex combs- (esc-) mutant embryos, the en expression pattern is nearly normal in esc- embryos. This suggests that the esc gene product functions in a pathway independent of the other genes in the group. The data indicate that the same group of genes is required for stable restriction of en expression to a striped pattern and for the restriction of expression of homeotic genes along the anterior-posterior axis, and support a global role for the Pc group genes in stable repression of activity of developmental selector genes.
Figures
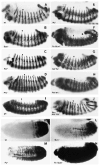
Fig. 1
Localization of en (A-J) and Abd-B (K-N) in wild-type and Pc group mutant embryos. The en protein and the Abd-B transcripts were detected as described in Materials and methods. In wild-type embryos (A), en is expressed in a series of stripes about 1-2 cells wide at this stage of development (∼9.5-10.5 hours; DiNardo et al., 1985). Mid-laterally a spur of en expression extends anteriorly. The first through the seventh abdominal segments (A1-A7) also contain a lone _en_-positive cell in their anterior domains; arrowheads provide a reference point between the third thoracic (T3) and the first abdominal (A1) segments. Mutant embryos (B-J) show different degrees of ectopic en expression (see text). Arrows in B and D point to examples of subtle ectopic expression. The embryo in H is about 20 minutes younger than the rest. Abd-B transcripts are expressed in parasegments 10-14 in wild type (K; Kuziora and McGinnis, 1988) and ectopically in the more anterior parasegments in mutant embryos (L-N). Representative mutant alleles are shown here: Pc3 (E,F,G, H,M,N), ScmXF24 (B,F,L,N), PclE90 (C,G), Psc1 (D), and Df (2R) vgD (H,J); AsxXF23 (J), ph503 (I).
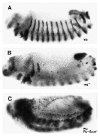
Fig. 2
_wg_-independent derepression of en. (A) wt; (B) _wg_−; (C) _wg_−; _Pc_−_Scm_−. The en protein was detected as described in Materials and methods. Embryos are the progeny of wgcx4/+;Pc3ScmXF24/+ heterozygotes.
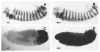
Fig. 3
Comparison of expression of en (A,B) and Abd-B (C,D) in _esc_− mutant embryos. Note that although en is not derepressed in _esc_− mutant embryos, the morphology of all stripes resembles that of an A8 stripe. _esc_− mutant embryos were collected from esc10/esc2 adult flies constructed as described in Materials and methods.
Fig. 4
Requirement for the Pc group in maintaining the off state of en and the homeotic genes suggests that Pc group function is uniformly present in the embryo. Ovals represent anterior (a) or posterior (p) cell nuclei of the second thoracic (T2) through the fifth abdominal (A5) segments in the epidermis along the anterior-posterior body axis. In A, each nucleus is divided into three parts, which designate the different homeotic loci that are simultaneously controlled by the Pc group. B shows the state of expression of en in nuclei corresponding to those in A. Shaded areas denote a locus under Pc group repression and unshaded areas show the regions where a locus is active. A schematic diagram depicting the graded repressor model as envisioned by Lewis (1978) is shown in A. All of the genes in the bithorax complex are inactive in the more anterior segments of the embryo (e.g. anterior T2) where repressor activity is high. Conversely, in the more posterior segments of the embryo (e.g. posterior A4), where repressor activity is low, all of the genes in the complex are active. As shown, the sensitivities of en (B) and the homeotic genes (A;Ubx, abd-A, and Abd-B) are independently controlled in a fashion that cannot be reconciled with genes having a different level of sensitivity to a common graded repressor. Ubx, Ultrabithorax; abd-A, abdominal-A; Abd-B, Abdominal-B.
Similar articles
- Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products.
Simon J, Chiang A, Bender W. Simon J, et al. Development. 1992 Feb;114(2):493-505. doi: 10.1242/dev.114.2.493. Development. 1992. PMID: 1350533 - The Drosophila extra sex combs protein contains WD motifs essential for its function as a repressor of homeotic genes.
Sathe SS, Harte PJ. Sathe SS, et al. Mech Dev. 1995 Jul;52(1):77-87. doi: 10.1016/0925-4773(95)00392-e. Mech Dev. 1995. PMID: 7577677 - Elements of the Drosophila bithorax complex that mediate repression by Polycomb group products.
Simon J, Chiang A, Bender W, Shimell MJ, O'Connor M. Simon J, et al. Dev Biol. 1993 Jul;158(1):131-44. doi: 10.1006/dbio.1993.1174. Dev Biol. 1993. PMID: 8101171 - Cell patterning in the Drosophila segment: engrailed and wingless antigen distributions in segment polarity mutant embryos.
van den Heuvel M, Klingensmith J, Perrimon N, Nusse R. van den Heuvel M, et al. Dev Suppl. 1993:105-14. Dev Suppl. 1993. PMID: 8049466 Review. - The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function.
Kennison JA. Kennison JA. Annu Rev Genet. 1995;29:289-303. doi: 10.1146/annurev.ge.29.120195.001445. Annu Rev Genet. 1995. PMID: 8825476 Review.
Cited by
- PRC2 functions in development and congenital disorders.
Deevy O, Bracken AP. Deevy O, et al. Development. 2019 Oct 1;146(19):dev181354. doi: 10.1242/dev.181354. Development. 2019. PMID: 31575610 Free PMC article. Review. - Isolation of cDNAs encoding the Drosophila GAGA transcription factor.
Soeller WC, Oh CE, Kornberg TB. Soeller WC, et al. Mol Cell Biol. 1993 Dec;13(12):7961-70. doi: 10.1128/mcb.13.12.7961-7970.1993. Mol Cell Biol. 1993. PMID: 7504178 Free PMC article. - Expression and properties of wild-type and mutant forms of the Drosophila sex comb on midleg (SCM) repressor protein.
Bornemann D, Miller E, Simon J. Bornemann D, et al. Genetics. 1998 Oct;150(2):675-86. doi: 10.1093/genetics/150.2.675. Genetics. 1998. PMID: 9755199 Free PMC article. - Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced.
Gindhart JG Jr, Kaufman TC. Gindhart JG Jr, et al. Genetics. 1995 Feb;139(2):797-814. doi: 10.1093/genetics/139.2.797. Genetics. 1995. PMID: 7713433 Free PMC article. - A 1-megadalton ESC/E(Z) complex from Drosophila that contains polycomblike and RPD3.
Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. Tie F, et al. Mol Cell Biol. 2003 May;23(9):3352-62. doi: 10.1128/MCB.23.9.3352-3362.2003. Mol Cell Biol. 2003. PMID: 12697833 Free PMC article.
References
- Allen ND, Norris ML, Surani MA. Epigenetic control of transgene expression and imprinting by genetic-specific modifiers. Cell. 1990;61:853–861. - PubMed
- Breen T, Duncan IM. Maternal expression of genes that regulate the bithorax complex of Drosophila melanogaster. Dev Biol. 1986;118:442–456. - PubMed
- Busturia A, Morata G. Ectopic expression of homeotic genes caused by the elimination of the Polycomb gene in Drosophila imaginal epidermis. Development. 1988;104:713–720. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials