Hypothalamic malonyl-CoA as a mediator of feeding behavior - PubMed (original) (raw)
Hypothalamic malonyl-CoA as a mediator of feeding behavior
Zhiyuan Hu et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Previous studies showed that i.p. administration of C75, a potent inhibitor of fatty acid synthase (FAS), blocked fasting-induced up-regulation of orexigenic neuropeptides and down-regulation of anorexigenic neuropeptides in the hypothalami of mice. As a result, food intake and body weight were drastically reduced. Here we provide evidence supporting the hypothesis that hypothalamic malonyl-CoA, a substrate of FAS, is an indicator of global energy status and mediates the feeding behavior of mice. We use a sensitive recycling assay to quantify malonyl-CoA to show that the hypothalamic malonyl-CoA level is low in fasted mice and rapidly (< or = 2 h) increases (approximately 5-fold) on refeeding. Intracerebroventricular (i.c.v.) administration of C75 to fasted mice rapidly (< or = 2 h) increased (by 4-fold) hypothalamic malonyl-CoA and blocked feeding when the mice were presented with food. Moreover, prior i.c.v. administration of an acetyl-CoA carboxylase inhibitor, 5-(tetradecyloxy)-2-furoic acid, rapidly (although only partially) prevented the C75-induced rise of hypothalamic malonyl-CoA and prevented the C75-induced decrease of food intake. These effects correlated closely with the rapid (< or = 2 h) and reciprocal effects of i.c.v. C75 on the expression of hypothalamic orexigenic (NPY and AgRP) and anorexigenic (proopiomelanocortin) neuropeptide mRNAs. Previous results showing that C75 administered i.c.v. rapidly activates hypothalamic neurons of the arcuate and paraventricular nuclei are consistent with the results reported in this paper. Together these findings suggest that level of hypothalamic malonyl-CoA, which depends on the relative activities of acetyl-CoA carboxylase and FAS, is an indicator of energy status and mediates feeding behavior.
Figures
Fig. 1.
Enzymatic recycling assay for malonyl-CoA. (A) Protocol for determination of hypothalamic malonyl-CoA by using bacterial malonyl-CoA decarboxylase. Acetyl-CoA in the tissue extract was first eliminated with citrate synthase in the presence of oxaloacetate. Then the cycling reaction was initiated by addition of excess malonate, ATP, and malonate decarboxylase, followed by the addition of acetate kinase. The malonate decarboxylase multicomponent enzyme first decarboxylates malonyl-CoA to produce acetyl-CoA and then transfers CoA from acetyl-CoA to malonate to regenerate malonyl-CoA. Repetition of these events amplifies accumulation of the ultimate product, acetate, which is then converted to acetyl-phosphate. After addition of hydroxylamine, the acetyl-hydroxamate formed is quantified at _A_540. (B) Standard curve for the malonyl-CoA assay. (C) Verification that contaminating acetyl-CoA does not interfere with quantification of malonyl-CoA in the recycling assay. An excess (60 pmol) of acetyl-CoA (to mimic acetyl-CoA contaminant in tissue extracts) was added (or not) to the reaction mixture with or without 2.5 pmol of malonyl-CoA in the presence or absence of citrate synthase. The amount of acetyl-hydroxamate formed (as indicated by _A_540) was not affected by added acetyl-CoA, verifying lack of interference in the recycling assay.
Fig. 2.
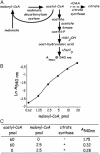
Long-term effects of C75 administered i.p. on blood glucose, hypothalamic malonyl-CoA, and food intake. Male BALB/c mice were acclimated for 1 week to a 12-h light/12-h dark cycle at 22°C and fed standard laboratory chow ad libitum. C75 or vehicle was administered by i.p. injection 2 h before the start of the dark cycle. Cumulative food intake over the next 24 h was measured. At that point, blood samples were drawn and hypothalami were quickly removed for malonyl-CoA analysis. Another group of mice was given mock injection and pair-fed the same quantity of food consumed by C75-treated mice. (A) Effect of C75 administered i.p. on 24-h food intake. (B) Effect of C75 administered i.p. on hypothalamic malonyl-CoA level. (C) Effect of C75 administered i.p. on blood glucose level. There were five mice per treatment. *, P < 0.001 vs. ad libitum; **, P < 0.001 vs. both ad libitum and C75.
Fig. 3.
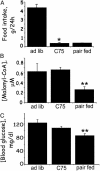
Short-term effects of C75 administered i.c.v. on blood glucose, hypothalamic malonyl-CoA, and food intake. Male BALB/c mice were acclimated for 1 week to a 12-h light/12-h dark cycle at 22°C and fed standard laboratory chow ad libitum. Mice were fasted for 23 h and then given 10 μg of C75 or vehicle by i.c.v. injection at 1.5 h before the start of the dark cycle. Thirty minutes after i.c.v. injection, food was presented to the mice and cumulative food intake was measured for 2 h. Hypothalami were then quickly removed for malonyl-CoA analysis. Another group of mice previously fasted for 23 h was given an i.c.v. injection of vehicle, and 2.5 h later hypothalami were removed for analysis. (A) Effect of C75 administered i.c.v. on 2-h food intake. (B) Effect of C75 administered i.c.v. on blood glucose level. (C) Effect of C75 administered i.c.v. on hypothalamic malonyl-CoA level. There were four mice per treatment. *, P < 0.001 vs. refed; **, P < 0.001 vs. both refed and C75.
Fig. 4.
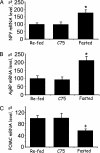
Short-term effects of C75 administered i.c.v. on the expression of orexigenic and anorexigenic neuropeptides in the hypothalamus. Mice were treated as described in Fig. 3. Two and a half hours after i.c.v. injection of C75, hypothalami were removed, and RNA was isolated and subjected to RNase protection analysis by using antisense probes specific for each neuropeptide mRNA. (A) NPY. (B) AgRP. (C) POMC. There were four mice per treatment. *, P < 0.001 vs. both refed and C75. +, P < 0.01 vs. C75 and P < 0.001 vs. refed. Results are presented as percentage relative to the level in refed mice.
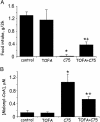
Fig. 5.
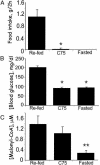
i.c.v. administration of an ACC inhibitor rapidly blocks i.c.v. C75-induced up-regulation of hypothalamic malonyl-CoA and suppression of food intake. Male BALB/c mice were first fasted for 24 h. Where indicated, TOFA (2 μg), an ACC inhibitor, or vehicle was injected i.c.v. 2.5 h before the dark cycle. Where indicated, C75 (10 μg) or vehicle was injected i.c.v. 1 h before the dark cycle. In some cases, both TOFA and C75 were injected sequentially. (A) To determine the effects of TOFA or C75 or both (TOFA, then C75) on food intake, mice were given food 0.5 h before the dark cycle, after which food consumption was measured during the next 2 h. (B) Hypothalami were removed for malonyl-CoA analysis 2.5 h after C75 or 4 h after TOFA injection. Mice for analysis of hypothalamic malonyl-CoA did not receive food. There were four mice per treatment. *, P < 0.001 vs. control or TOFA alone; +, P < 0.001 vs. C75.
Fig. 6.
Model for hypothalamic malonyl-CoA as mediator of expression of orexigenic and anorexigenic neuropeptides and food intake.
Similar articles
- Effect of centrally administered C75, a fatty acid synthase inhibitor, on ghrelin secretion and its downstream effects.
Hu Z, Cha SH, van Haasteren G, Wang J, Lane MD. Hu Z, et al. Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):3972-7. doi: 10.1073/pnas.0500619102. Epub 2005 Feb 23. Proc Natl Acad Sci U S A. 2005. PMID: 15728730 Free PMC article. - Effect of a fatty acid synthase inhibitor on food intake and expression of hypothalamic neuropeptides.
Shimokawa T, Kumar MV, Lane MD. Shimokawa T, et al. Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):66-71. doi: 10.1073/pnas.012606199. Epub 2001 Dec 26. Proc Natl Acad Sci U S A. 2002. PMID: 11756683 Free PMC article. - Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA.
Lane MD, Wolfgang M, Cha SH, Dai Y. Lane MD, et al. Int J Obes (Lond). 2008 Sep;32 Suppl 4:S49-54. doi: 10.1038/ijo.2008.123. Int J Obes (Lond). 2008. PMID: 18719599 Review. - Effect of the anorectic fatty acid synthase inhibitor C75 on neuronal activity in the hypothalamus and brainstem.
Gao S, Lane MD. Gao S, et al. Proc Natl Acad Sci U S A. 2003 May 13;100(10):5628-33. doi: 10.1073/pnas.1031698100. Epub 2003 Apr 30. Proc Natl Acad Sci U S A. 2003. PMID: 12724522 Free PMC article. - Role of malonyl-CoA in heart disease and the hypothalamic control of obesity.
Folmes CD, Lopaschuk GD. Folmes CD, et al. Cardiovasc Res. 2007 Jan 15;73(2):278-87. doi: 10.1016/j.cardiores.2006.10.008. Epub 2006 Oct 20. Cardiovasc Res. 2007. PMID: 17126822 Review.
Cited by
- Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats.
Martínez de Morentin PB, Lage R, González-García I, Ruíz-Pino F, Martins L, Fernández-Mallo D, Gallego R, Fernø J, Señarís R, Saha AK, Tovar S, Diéguez C, Nogueiras R, Tena-Sempere M, López M. Martínez de Morentin PB, et al. Endocrinology. 2015 Mar;156(3):947-60. doi: 10.1210/en.2014-1611. Epub 2014 Dec 23. Endocrinology. 2015. PMID: 25535827 Free PMC article. - AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity.
Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jo H, Jeong KH, Tadi S, Park MG, Kwak TH, Man Kim J, Hyun DH, Kim JB. Lee JW, et al. J Lipid Res. 2012 Jul;53(7):1277-86. doi: 10.1194/jlr.M022897. Epub 2012 Apr 9. J Lipid Res. 2012. PMID: 22493094 Free PMC article. - Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake.
Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Gao S, et al. Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17358-63. doi: 10.1073/pnas.0708385104. Epub 2007 Oct 23. Proc Natl Acad Sci U S A. 2007. PMID: 17956983 Free PMC article. - The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis.
Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, Lane MD. Wolfgang MJ, et al. Proc Natl Acad Sci U S A. 2006 May 9;103(19):7282-7. doi: 10.1073/pnas.0602205103. Epub 2006 May 1. Proc Natl Acad Sci U S A. 2006. PMID: 16651524 Free PMC article. - Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin.
Zhao J, Sun XB, Ye F, Tian WX. Zhao J, et al. Mol Cell Biochem. 2011 May;351(1-2):19-28. doi: 10.1007/s11010-010-0707-z. Epub 2011 Jan 9. Mol Cell Biochem. 2011. PMID: 21221723
References
- Schwartz, M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404 661–671. - PubMed
- Williams, G., Bing, C., Cai, X. J., Harrold, J. A., King, P. J. & Liu, X. H. (2001) Physiol. Behav. 74 683–701. - PubMed
- Morton, G. J. & Schwartz, M. W. (2001) Int. J. Obes. Relat. Metab. Disord. 25 Suppl. 5, S56–S62. - PubMed
- Mercer, J. G. & Speakman, J. R. (2001) Neurosci. Biobehav. Rev. 25 101–116. - PubMed
- Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D. & Kuhajda, F. P. (2000) Science 288 2379–2381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous