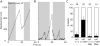Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation - PubMed (original) (raw)
Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation
Grégory Giannone et al. J Cell Biol. 2003.
Abstract
Cells rapidly transduce forces exerted on extracellular matrix contacts into tyrosine kinase activation and recruitment of cytoskeletal proteins to reinforce integrin-cytoskeleton connections and initiate adhesion site formation. The relationship between these two processes has not been defined, particularly at the submicrometer level. Using talin1-deficient cells, it appears that talin1 is critical for building early mechanical linkages. Deletion of talin1 blocked laser tweezers, force-dependent reinforcement of submicrometer fibronectin-coated beads and early formation of adhesion sites in response to force, even though Src family kinases, focal adhesion kinase, and spreading were activated normally. Recruitment of vinculin and paxillin to sites of force application also required talin1. FilaminA had a secondary role in strengthening fibronectin-integrin-cytoskeleton connections and no role in stretch-dependent adhesion site assembly. Thus, force-dependent activation of tyrosine kinases is independent of early force-dependent structural changes that require talin1 as part of a critical scaffold.
Figures
Figure 1.
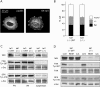
Integrin- and force-dependent activation of SFKs and FAK is normal during spreading of talin1-deficient cells on FN. (A) After 30 min of spreading on FN 120 kD, talin1 (−/−) cells transiently cotransfected with HA-talin1 and paxillin-GFP were fixed; paxillin-GFP and HA-talin1 were visualized by fluorescence and immunofluorescence, respectively. (B) After 10 min of spreading on FN 120 kD, talin1 (−/−) cells or cells transiently cotransfected with talin1 and EGFP (talin1 (−/−)WT) cells were scored for flat, intermediary, or round morphology. Results represent the mean ± SD of three experiments. (C) Talin1 (−/−) and talin1 (−/−)WT cell suspension or cells allowed to spread for 10 min on either FN 120 kD or VN were lysed, and the protein was analyzed by Western blotting using a phosphospecific anti-SFK (SFK Tyr-416), a phosphospecific anti-FAK (FAK Tyr-397), and a talin antibody. The total amount of proteins was verified using an anti-Src antibody. (D) Talin1 (−/−) and talin1 (−/−)WT cells allowed to spread for 10 min on FN in the presence or absence (cont) of 20 mM of the myosin inhibitor BDM or in suspension (sus) were lysed; the protein was analyzed by Western blotting using a phosphospecific anti-SFK (SFK Tyr-416), a phosphospecific anti-FAK (FAK Tyr-397), and a talin antibody. The total amount of proteins was verified using an anti-Src and an anti-FAK antibody. (C and D) Results shown are representative of three independent experiments.
Figure 2.
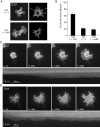
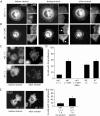
The initiation and stabilization of focal complexes to focal adhesions is delayed in talin1-deficient cells. Talin1 (−/−), talin1 (−/−)WT, and talin1 (−/−)ABS were transiently transfected with paxillin-GFP. After 1 h of spreading on FN 120 kD–coated coverslips, cells were fixed, and paxillin-GFP was visualized (A) to quantify the percentage of cells with focal adhesions (B). Approximately 65% of talin1 (−/−)WT cells expressing full-length talin1 displayed paxillin-positive adhesions (A, bottom right) compared with only 20% in talin1 (−/−) (A, top right) and talin1 (−/−)ABS cells (B). Results represent the mean ± SD of at least three experiments. (C) Formation of adhesion sites was observed in talin1 (−/−)WT cells transfected with paxillin-GFP spreading on FN 120 kD–coated coverslips using TIRF microscopy. The bottom kymograph of the dotted box displayed in the upper sequence showed that the initiation and stabilization of focal complexes is fast and simultaneous with protrusion/retraction of the lamellipodia in talin1 (−/−)WT cells. (D) In talin1 (−/−) cells transfected with paxillin-GFP spreading, no distinct adhesion sites were formed during >30-min acquisition time. The bottom kymograph of the dotted box displayed in the upper sequence showed that despite extensive protrusion/retraction events of the lamellipodia, no initiation of focal complexes was observed.
Figure 3.
FNIII7–10–integrin–talin1–actin connections are essential for the force-dependent strengthening of integrin–cytoskeleton linkages. (A) A representative trace showing displacement of trimeric FNIII7–10-coated beads from their initial position over time on the surface of talin1 (−/−)WT cells. Restrained beads (gray strips, turn on of the laser trap) able to escape the laser trap (movement outside the first gray strip) were tested with a second pulse (second gray strip) after turning off (white strip) and repositioning the laser trap (<0.5 μm behind the bead). Beads were scored reinforced if they were unable to be dislocated (no changes of the bead trajectory >100 nm, just after the beginning of the second gray strip, test pulse). Most beads were not displaced by the test pulse (retrap [reinforced]), suggesting that the cell regulates, in response to the rigidity of the laser trap, the strength of integrin–cytoskeleton linkages. (B) Representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of talin1 (−/−) cells. Trimeric FNIII7–10-coated beads, which were not reinforced, were displaced by the laser trap test pulse (second gray strip; retrap [loose]) after initial escape from the laser trap (movement outside the first gray strip). (C) Summary of reinforcement assay results, showing the percentage of experiments in which beads were reinforced (black bars) or loose (white bars). Transfected cells were identified by EGFP cotransfection. Results represent the mean ± SD of at least three experiments.
Figure 4.
Talin1 is necessary for fast stabilization of integrin–cytoskeleton linkages after application of force on FNIII7–10–integrin–cytoskeleton linkages. (A) Representative DIC image of trimeric FNIII7–10-coated beads restrained by the laser trap just after positioning on the surface of the lamellipodia of a talin1 (−/−) cell, and superimposed are the x and y coordinates (blue trace) of the bead movement. The graph shows the parallel and perpendicular movement of the bead while escaping from the laser trap (x and y coordinates, respectively). The movement pattern is characterized by first, a stabilization of the bead in the laser trap center, followed by a linear movement until the bead escapes the laser force field where it undergoes a more diffuse movement. (B) Representative DIC image of trimeric FNIII7–10-coated beads restrained by the laser trap just after positioning on the surface of the lamellipodia of a talin1 (−/−)WT cell and the superimposed x and y coordinates (blue trace) of the bead. The graph shows that compared with talin1 (−/−) cells, coated beads displayed a direct linear movement out of the laser trap, and that the movement remained linear after the bead escaped the laser force field. (C) Summary of mean square displacement (MSD) quantification after beads left the trap field (500 nm; see Materials and methods). Results shown are the mean ± SD of at least eight independent experiments (ANOVA, P > 0.05).
Figure 5.
Force-dependent stabilization of integrin–cytoskeleton linkages involves recruitment of paxillin and vinculin by talin1. (A) Talin1 (−/−)WT (top) and talin1 (−/−) (bottom) cells were transfected with paxillin-GFP and were allowed to spread for 30 min. Large FNIII7–10-coated beads (5.9-μm diam) were spun onto the cells and incubated for 30 min. (left) Paxillin-GFP; (right) merged fluorescence and DIC images showing bead-induced accumulation of paxillin-GFP. (B) Talin1 (−/−)WT (top) and talin1 (−/−) (bottom) cells were transfected with EGFP-vinculin, and the cells were treated with FNIII7–10-coated beads as in A. (left) EGFP-vinculin; (right) merged fluorescence and DIC images, showing bead-induced accumulation of EGFP-vinculin. (C) Percentage of cells displaying accumulation of paxillin or vinculin at the bead interface. Cells were scored positive if accumulation of paxillin-GFP or EGFP-vinculin was detected beneath any bead bound within 10 μm from the edge. Results shown are the mean ± SD of three independent experiments.
Figure 6.
FilaminA is moderately involved in fast stabilization of integrin–cytoskeleton linkages. (A) A representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of filaminA-expressing A7 cells. (B) Representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of filaminA-null M2 cells. (A and B) Gray and white strips represent turn on and off of the laser trap, respectively (Fig. 3). (C) Summary of reinforcement assay results showing the percentage of experiments in which beads were reinforced (black bars) or loose (white bars). FilaminA-deficient M2 melanoma cells displayed a relatively weak impairment of the reinforcement process compared with A7 cells expressing the filaminA cDNA. Results represent the mean ± SD of at least three experiments. (D) Summary of MSD quantification after beads left the trap field (500 nm; see Materials and methods). Results shown are the mean ± SD of at least five independent experiments.
Figure 7.
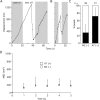
Talin1 is critical for the early integrin- and stretch-dependent formation of adhesion sites. (A) Representative stretching (10%) of talin1 (−/−) cells transiently transfected with paxillin-GFP. After 10 min of spreading, cells were stretched, held for 2 min, and subsequently relaxed by allowing the stretched silicone membrane to return to its original size. Before stretch (left), 2 min after stretch (middle), and after relaxation of stretch (right). Boxed areas are shown enlarged (right panels). (B) Representative stretching of talin1 (−/−) cells transiently transfected with the full-length talin1 and paxillin-GFP. As for talin1 (−/−) cells, no adhesion sites were visible in talin1 (−/−) WT cells before stretch (left). However, after 2-min stretch, adhesion sites were formed at the cell periphery visualized by paxillin-GFP fluorescence (arrows). Boxed areas are shown enlarged (right panels). (C) M2 (filaminA null) or A7 cells (stably expressing filaminA) were allowed to spread for 10 min and stretched for 2 min before fixation with 3.7% formaldehyde/PBS. M2 and A7 cells were subjected to immunostaining using antipaxillin antibody. (D) Summary of stretch-dependent formation of adhesion sites experiments during cell spreading. (E) Talin1 (−/−) cells transiently transfected with paxillin-GFP were allowed to spread for 1 h on pronectin-coated silicone membranes, and were stretched or not for 2 min before fixation. Representative localization of paxillin-GFP in talin1 (−/−) cells before stretch (left) and after stretch (right). (F) Summary of stretch-dependent formation of adhesion sites experiments after 1 h of cell spreading. (D and F) Results shown are the mean ± SD of at least three independent experiments.
Figure 8.
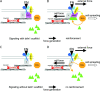
Talin1 acts as a scaffold but does not support the signaling in the reinforcement of integrin–cytoskeleton interactions. (A) FN binding to αvβ3/integrin induces the rapid formation of a weak slipping connection between talin1 and the rearward moving cytoskeleton. (B) Sustained force applied on the FN–integrin–cytoskeleton connection induces αvβ3/integrin-dependent activation of RPTPα/SFKs, which is responsible for paxillin and vinculin (green triangles) recruitment to the talin1/cytoskeletal interface. Assembly of this complex leads to stabilization of the talin1–cytoskeleton connection and is responsible for focal complex initiation and stabilization. (C) In the absence of talin1, protein X is envisaged to support the delayed interaction of integrins with the actin cytoskeleton. (D) Protein X supports the force-induced activation of RPTPα/SFKs, but this does not result in efficient recruitment of paxillin and vinculin, and this precludes reinforcement of the initial linkage. The interaction of integrins with the actin cytoskeleton is not stabilized and focal complexes fail to form. In either case, RPTPα–SFK pathway activation is involved in stimulation of cell spreading.
Similar articles
- Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin.
Carragher NO, Levkau B, Ross R, Raines EW. Carragher NO, et al. J Cell Biol. 1999 Nov 1;147(3):619-30. doi: 10.1083/jcb.147.3.619. J Cell Biol. 1999. PMID: 10545505 Free PMC article. - Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL.
Salgia R, Brunkhorst B, Pisick E, Li JL, Lo SH, Chen LB, Griffin JD. Salgia R, et al. Oncogene. 1995 Sep 21;11(6):1149-55. Oncogene. 1995. PMID: 7566975 - NADPH oxidase activation in fibronectin adherent human neutrophils: A potential role for beta1 integrin ligation.
Umanskiy K, Robinson C, Cave C, Williams MA, Lentsch AB, Cuschieri J, Solomkin JS. Umanskiy K, et al. Surgery. 2003 Aug;134(2):378-83. doi: 10.1067/msy.2003.253. Surgery. 2003. PMID: 12947344 - Focal adhesions - the cytoskeletal connection.
Critchley DR. Critchley DR. Curr Opin Cell Biol. 2000 Feb;12(1):133-9. doi: 10.1016/s0955-0674(99)00067-8. Curr Opin Cell Biol. 2000. PMID: 10679361 Review. - The interplay between Src and integrins in normal and tumor biology.
Playford MP, Schaller MD. Playford MP, et al. Oncogene. 2004 Oct 18;23(48):7928-46. doi: 10.1038/sj.onc.1208080. Oncogene. 2004. PMID: 15489911 Review.
Cited by
- The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening.
Hersch N, Wolters B, Dreissen G, Springer R, Kirchgeßner N, Merkel R, Hoffmann B. Hersch N, et al. Biol Open. 2013 Mar 15;2(3):351-61. doi: 10.1242/bio.20133830. Epub 2013 Jan 30. Biol Open. 2013. PMID: 23519595 Free PMC article. - Mechano-sensing by actin filaments and focal adhesion proteins.
Hayakawa K, Tatsumi H, Sokabe M. Hayakawa K, et al. Commun Integr Biol. 2012 Nov 1;5(6):572-7. doi: 10.4161/cib.21891. Commun Integr Biol. 2012. PMID: 23336027 Free PMC article. - Integrins contribute to initial morphological development and process outgrowth in rat adult hippocampal progenitor cells.
Harper MM, Ye EA, Blong CC, Jacobson ML, Sakaguchi DS. Harper MM, et al. J Mol Neurosci. 2010 Mar;40(3):269-83. doi: 10.1007/s12031-009-9211-x. Epub 2009 Jun 5. J Mol Neurosci. 2010. PMID: 19499350 - Forces generated by lamellipodial actin filament elongation regulate the WAVE complex during cell migration.
Mehidi A, Kage F, Karatas Z, Cercy M, Schaks M, Polesskaya A, Sainlos M, Gautreau AM, Rossier O, Rottner K, Giannone G. Mehidi A, et al. Nat Cell Biol. 2021 Nov;23(11):1148-1162. doi: 10.1038/s41556-021-00786-8. Epub 2021 Nov 4. Nat Cell Biol. 2021. PMID: 34737443
References
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. - PubMed
- Boettiger, D., L. Lynch, S. Blystone, and F. Huber. 2001. Distinct ligand-binding modes for integrin alpha(v)beta(3)-mediated adhesion to fibronectin versus vitronectin. J. Biol. Chem. 276:31684–31690. - PubMed
- Bolton, S.J., S.T. Barry, H. Mosley, B. Patel, B.M. Jockusch, J.M. Wilkinson, and D.R. Critchley. 1997. Monoclonal antibodies recognizing the N- and C-terminal regions of talin disrupt actin stress fibers when microinjected into human fibroblasts. Cell Motil. Cytoskeleton. 36:363–376. - PubMed
- Brown, N.H., S.L. Gregory, W.L. Rickoll, L.I. Fessler, M. Prout, R.A. White, and J.W. Fristrom. 2002. Talin is essential for integrin function in Drosophila. Dev. Cell. 3:569–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous