Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes - PubMed (original) (raw)
Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes
Sharron X Lin et al. Mol Biol Cell. 2004 Feb.
Abstract
Although the distribution of the cation-independent mannose 6-phosphate receptor (CI-MPR) has been well studied, its intracellular itinerary and trafficking kinetics remain uncertain. In this report, we describe the endocytic trafficking and steady-state localization of a chimeric form of the CI-MPR containing the ecto-domain of the bovine CI-MPR and the murine transmembrane and cytoplasmic domains expressed in a CHO cell line. Detailed confocal microscopy analysis revealed that internalized chimeric CI-MPR overlaps almost completely with the endogenous CI-MPR but only partially with individual markers for the trans-Golgi network or other endosomal compartments. After endocytosis, the chimeric receptor first enters sorting endosomes, and it then accumulates in the endocytic recycling compartment. A large fraction of the receptors return to the plasma membrane, but some are delivered to the trans-Golgi network and/or late endosomes. Over the course of an hour, the endocytosed receptors achieve their steady-state distribution. Importantly, the receptor does not start to colocalize with late endosomal markers until after it has passed through the endocytic recycling compartment. In CHO cells, only a small fraction of the receptor is ever detected in endosomes bearing substrates destined for lysosomes (kinetically defined late endosomes). These data demonstrate that CI-MPR takes a complex route that involves multiple sorting steps in both early and late endosomes.
Figures
Figure 10.
Schematic model of CI-MPR trafficking in CHO cells. The model compares the postendocytic itineraries of the CI-MPR (MR) and its enzyme ligands (E) with the transferrin receptor (TR), transferrin (Tf) with or without iron (Fe), the LDL receptor (LR), LDL, furin (F), and TGN38 (T). All of the membrane proteins concentrate into clathrin-coated pits, and the initial delivery site is sorting endosomes. Most of the membrane proteins, including CI-MPR, rapidly exit this compartment and are either returned directly to the plasma membrane (PM) or are transported to the ERC. Furin is retained in the sorting endosome as it begins to mature into a late endosome, and furin reaches the Golgi via the late endosomes. From the ERC, essentially all of the transferrin receptors recycle to the cell surface, and ∼85% of the internalized TGN38 and CI-MPR also return to the cell surface. The remaining TGN38 and CI-MPR are delivered to the _trans_-Golgi. The CI-MPR traffics from the _trans_-Golgi to late endosomes, where the enzyme ligands dissociate as a consequence of exposure to a sufficiently acidic pH. Furin and the empty CI-MPR return to the _trans_-Golgi. It is not known if furin and CI-MPR share the same transport vesicle as they shuttle between late endosomes and the _trans_-Golgi. A portion of all of the molecules in the _trans_-Golgi is delivered to the cell surface.
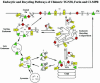
Figure 1.
At steady state, the chimeric CI-MPR completely codistributes with endogenous CI-MPR and localizes to endosomes and the TGN/Golgi. TRVb-1 cells were fixed, permeabilized, and labeled with Alexa488-anti-bCI-MPR and polyclonal antibodies against endogenous CI-MPR (A, Endog MPR), TGN38 (B), β-COP (C, COP), and LAMP-2 (E), followed by Cy3-anti-rabbit secondary antibodies. In F, TRVb-1 cells were incubated with 10 mg/ml rhodamine-dextran (70 kDa, fixable) for 60 min, which labels mostly late endosomes and lysosomes, and then fixed. TRVb-1 cells expressing the CI-MPR chimera were incubated for 60 min with Cy3-transferrin (G) or with Cy3-anti-bCI-MPR for 2 h (D and H). The cells that received the anti-MPR in the medium were fixed and labeled with C6-NBD-ceramide (D, NBD-C6 Cer) to mark the TGN or with antibodies against the clathrin adaptor AP1 (H). Shown are confocal Z-series projections. For simplicity, chimeric MPR is pseudocolored in green in all panels. Bars, 5 μm.
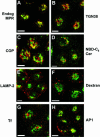
Figure 2.
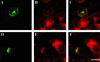
Endogenous hamster CI-MPR colocalizes partially with internalized Tf. TRVb-1 cells not expressing recombinant CI-MPR (A-D) were incubated for 5 min with 5 μg/ml Cy3-Tf (B, in A-B), and then fixed, permeabilized, and stained with 5 μg/ml Alexa488-anti-bCI-MPR (A). Arrows point to peripheral endosomes labeled for both molecules. Z series confocal projections are shown. In C and D, cells were incubated with 20 μg/ml Cy5-LDL for 5 min, and then washed and chased in medium for 10 or 20 min before fixation. Cells were then permeabilized and stained with anti-MPR antibody followed by Cy3-antirabbit secondary antibody. LDL is pseudocolored in red and MPR in green in both panels. Z-series confocal projections are shown. Bars, 5 μm.
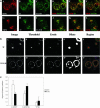
Figure 3.
Most of the chimeric CI-MPR is found in the endocytic recycling compartment and the TGN. In a, TRVb-1 cells expressing the chimeric CI-MPR were incubated with Cy3-anti-bCI-MPR and Alexa488-Tf for 30 min (A-F) and followed by chase for 30 min (G-L) in the absence of Cy3-MPR but in the presence of Alexa488-Tf. Cells were then fixed, permeabilized, and stained with anti-TGN38 antibody, followed with Cy5-goat anti-rabbit secondary antibody (C and I). The Cy3-anti-bCI-MPR staining is shown in A and G, the Alexa488-Tf staining is shown in B and H and the TGN38 antibody staining is shown in C and I. For the convenience of direct comparison, TGN38 staining was pseudocolored in green. In D and J, the distribution of CI-MPR (red) was compared with that of both Tf and TGN38 (both are green). In E and F and K and L, the distribution of MPR (red) was compared with Tf (green in E and K) and TGN38 (green in F and L). Bars, 10 μm. In b, the method to generate the regions of interest for the measurement of regional r is illustrated. First, images of the organelle marker (transferrin or TGN38) were thresholded to define the fluorescent probe-labeled regions of interest. The thresholded objects were eroded by 6 pixels to eliminate small objects, and the remaining objects were dilated by 20 pixels to include the labeled organelle and the surrounding region. These regions were then used as a mask of the internalized CI-MPR channel for the r measurement. Results of these measurements and analysis based on seven images containing ∼29 cells are shown in the Figure 3C.
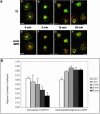
Figure 4.
The chimeric CI-MPR enters the endocytic recycling compartment after internalization, and then rapidly exits the recycling pathway and reaches steady state. In a, cells were incubated for 5 min with Alexa488-anti-bCI-MPR and with Cy5-Tf (A-D) or without Cy5-Tf (E-H). Cells were then washed and fixed (A and E) or chased for 5, 10, and 20 min in medium containing Cy5-Tf (B-D) or without Cy5-Tf (F-H) before fixation. In E-H, cells were permeabilized and stained with antibodies against the endogenous CI-MPR followed by Cy3-labeled secondary antibodies. The chimeric CI-MPR is pseudocolored in green in all panels. Cy5-Tf is pseudocolored red in A-D; endogenous CIMPR (endo MPR) is red in E-H. Z series confocal projections are shown. Bars, 5 μm. In b, the method illustrated in Figure 3B was used to generate the regions of interest for the measurement of regional r. First, images of the organelle marker (transferrin or endogenous CI-MPR) were thresholded to define the fluorescent probe-labeled regions of interest. The thresholded objects were eroded by 6 pixels to eliminate small objects, and the remaining objects were dilated by 20 pixels to include the labeled organelle and the surrounding region. These regions were then used as a mask of the internalized CI-MPR channel for the regional r measurement, which is based on the intensity values in the identical pixels in the two images. Results of these measurements and analysis based on 11 images containing ∼47 cells are shown in the Figure 4b.
Figure 5.
Expressing mutant mRme-1 G429R slows the exit of chimeric MPR from the ERC and decreases the receptor recycling to the cell surface. TRVb-1 cells stably transfected with chimeric CI-MPR were transiently transfected with FLAG-mRme-1 G429R. Cells were incubated with Cy3-anti-bCI-MPR for 30 min (A-C) followed by chased in the absence of Cy3-anti-bCI-MPR for an additional 30 min (D-F). In A-C, Alexa488-Tf (A) was preincubated with cells for 15 min and chased in the absence of Alexa488-Tf but in the presence of excess unlabeled Tf for 15 min before and during the incubation of Cy3-anti-bCIMPR for 30 min (B). The total chase time for Tf is 45 min. In D-E, Alexa488-Tf (D) was incubated together with Cy3-anti-bCI-MPR (E) for 15 min and was chased for 45 min. The merged images of A and B and D and E are shown in C and F, respectively. Z series confocal projections are shown. Bars, 10 μm.
Figure 6.
The chimeric CI-MPR showed limited codistribution with late endosomal probes. TRVb-1 cells expressing recombinant chimeric CI-MPR were incubated with 5 μg/ml Alexa488-anti-bCI-MPR (green) and 20 μg/ml DiI-LDL (red) for 5 min, and then were washed and fixed (A) or chased for 5 (B), 10 (C), or 20 (D) min before fixation. Shown are confocal Z-series projections. Bars, 5 μm.
Figure 7.
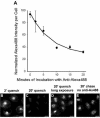
A substantial fraction of internalized CI-MPR is rapidly recycled to the plasma membrane through endosomes. (A) Cells were incubated for 3 min with 5 μg/ml Alexa488-anti-bCI-MPR, and then washed briefly. Cells were incubated with 10 μg/ml anti-Alexa488 for 2, 5, 10, 15, or 20 min before fixation. To obtain the zero-minute time point, cells were fixed after the Alexa488-antibCI-MPR pulse and incubated with anti-Alexa488 to quench antibody that had not yet been internalized. Unquenched fluorescence was detected by wide-field microscopy. Relative fluorescence intensities per cell are plotted as a function of time of incubation with anti-Alexa488. Data have been fitted to a mono-exponential decline. (B-E) Representative images from such an experiment, showing Alexa488 fluorescence after 2 min (B) or 20 min (C) with anti-Alexa488, or 20-min chase without anti-Alexa488 (E). (D) Enhanced copy of Panel C to illustrate the distribution of unquenched anti-bCI-MPR. Bars, 5 μm.
Figure 8.
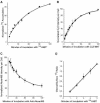
Kinetics of externalization and internalization of chimeric MPR. (A) Cells were incubated for 5, 10, 15, 20, 30, 45, 60, 90, and 120 min with 5 μg/ml125I-anti-bCI-MPR. Cells were washed with medium and dissolved in 0.1% SDS + 0.1 M NaOH. Solutes were collected and radioactivity determined using a gamma counter. Nonspecific binding to TRVb-1 cells not expressing the recombinant receptor was subtracted from the data. Data were fitted to a monoexponential increase. (B) Cells were incubated for 5, 10, 15, 20, 30, 45, 60, 90, and 120 min with 5 μg/ml Cy3-anti-bCI-MPR. Cells were washed, fixed, and imaged by epifluorescence microscopy, imaging 10 fields per time point. Data were fitted to a monoexponential increase. (C) Cells were incubated for 60 min with 5 μg/ml Alexa488-anti-bCI-MPR, and then washed and chased for 5, 10, 15, 20, 30, 45, 60, 90, and 120 min with 10 μg/ml anti-Alexa488. Cells were washed, fixed, and imaged by epifluorescence microscopy, imaging 10 fields per time point. Data were fitted to a monoexponential decline. (D) Cells received 5 μg/ml125I-anti-bCI-MPR for 1, 2, 3, 4, and 5 min at 37°C. Cells were immediately placed on ice and rapidly washed with cold buffer. Surface-bound 125I was removed by 2 × 10-min incubations at pH 2 at 4°C. Internalized125I was recovered by dissolution in 0.1% SDS + 0.1 M NaOH. Shown is the ratio of internal to surface-bound counts as a function of time. Data were fitted to a straight line; the slope gives the internalization rate. For each experiment, time points were taken in triplicate. For all panels, data are the averages of three independent experiments. Error bars are SEs of the mean.
Figure 9.
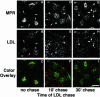
Chimeric CI-MPR appears in LDL-labeled late endosomes at a late stage in its endocytic trafficking. The cells were incubated with 5 μg/ml Alexa488-anti-bCI-MPR for 30 min followed by a 90-min chase. At the end of the chase, the cells were incubated for 5 min with 20 μg/ml DiI-LDL, washed, and fixed immediately (A-C) or chased for 10 (D-F) or 30 min (G-I) before fixation, such that for all samples the anti-MPR was chased for 90 min. In C, F, and I, internalized anti-bCI-MPR is colored green and LDL is colored red. Arrows in A and B indicate anti-bCI-MPR-labeled spots that do not label with LDL. Arrows in A, B, D, and E indicate nonoverlapping spots, and arrowheads in D, E, G, and H indicate double-labeled spots. Most CI-MPR is detected in a pericentriolar distribution, and the images have been enhanced to emphasize the dimmer punctate MPR labeling. Confocal Z-series projections are shown. Bars, 10 μm.
Similar articles
- Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor.
Nicoziani P, Vilhardt F, Llorente A, Hilout L, Courtoy PJ, Sandvig K, van Deurs B. Nicoziani P, et al. Mol Biol Cell. 2000 Feb;11(2):481-95. doi: 10.1091/mbc.11.2.481. Mol Biol Cell. 2000. PMID: 10679008 Free PMC article. - Overexpression of Rab22a hampers the transport between endosomes and the Golgi apparatus.
Mesa R, Magadán J, Barbieri A, López C, Stahl PD, Mayorga LS. Mesa R, et al. Exp Cell Res. 2005 Apr 1;304(2):339-53. doi: 10.1016/j.yexcr.2004.11.017. Epub 2004 Dec 16. Exp Cell Res. 2005. PMID: 15748882 - Role of an acidic cluster/dileucine motif in cation-independent mannose 6-phosphate receptor traffic.
Tortorella LL, Schapiro FB, Maxfield FR. Tortorella LL, et al. Traffic. 2007 Apr;8(4):402-13. doi: 10.1111/j.1600-0854.2007.00541.x. Epub 2007 Feb 23. Traffic. 2007. PMID: 17319895 - Post-Golgi traffic in plants.
Richter S, Voss U, Jürgens G. Richter S, et al. Traffic. 2009 Jul;10(7):819-28. doi: 10.1111/j.1600-0854.2009.00916.x. Epub 2009 Apr 18. Traffic. 2009. PMID: 19416470 Review. - Multiple routes of protein transport from endosomes to the trans Golgi network.
Pfeffer SR. Pfeffer SR. FEBS Lett. 2009 Dec 3;583(23):3811-6. doi: 10.1016/j.febslet.2009.10.075. Epub 2009 Oct 29. FEBS Lett. 2009. PMID: 19879268 Free PMC article. Review.
Cited by
- SMAP2 regulates retrograde transport from recycling endosomes to the Golgi.
Matsudaira T, Uchida Y, Tanabe K, Kon S, Watanabe T, Taguchi T, Arai H. Matsudaira T, et al. PLoS One. 2013 Jul 8;8(7):e69145. doi: 10.1371/journal.pone.0069145. Print 2013. PLoS One. 2013. PMID: 23861959 Free PMC article. - Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor.
Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Arighi CN, et al. J Cell Biol. 2004 Apr;165(1):123-33. doi: 10.1083/jcb.200312055. J Cell Biol. 2004. PMID: 15078903 Free PMC article. - PKAN hiPS-Derived Astrocytes Show Impairment of Endosomal Trafficking: A Potential Mechanism Underlying Iron Accumulation.
Ripamonti M, Santambrogio P, Racchetti G, Cozzi A, Di Meo I, Tiranti V, Levi S. Ripamonti M, et al. Front Cell Neurosci. 2022 Jun 16;16:878103. doi: 10.3389/fncel.2022.878103. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35783094 Free PMC article. - Molecular regulation of the plasma membrane-proximal cellular steps involved in NK cell cytolytic function.
Phatarpekar PV, Billadeau DD. Phatarpekar PV, et al. J Cell Sci. 2020 Feb 21;133(5):jcs240424. doi: 10.1242/jcs.240424. J Cell Sci. 2020. PMID: 32086255 Free PMC article. Review. - Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin.
Ikonomov OC, Fligger J, Sbrissa D, Dondapati R, Mlak K, Deeb R, Shisheva A. Ikonomov OC, et al. J Biol Chem. 2009 Feb 6;284(6):3750-61. doi: 10.1074/jbc.M806539200. Epub 2008 Dec 4. J Biol Chem. 2009. PMID: 19056739 Free PMC article.
References
- Borden, L.A., Einstein, R., Gabel, C.A., and Maxfield, F.R. (1990). Acidification-dependent dissociation of endocytosed insulin precedes that of endocytosed proteins bearing the mannose 6-phosphate recognition marker. J. Biol. Chem. 265, 8497-8504. - PubMed
- Bosshart, H., Humphrey, J., Deignan, E., Davidson, J., Drazba, J., Yuan, L., Oorschot, V., Peters, P.J., and Bonifacino, J.S. (1994). The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J. Cell Biol. 126, 1157-1172. - PMC - PubMed
- Chen, H.J., Remmler, J., Delaney, J.C., Messner, D.J., and Lobel, P. (1993). Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor. J. Cell Biol. 268, 22338-22346. - PubMed
- Chen, H.J., Yuan, J., and Lobel, P. (1997). Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. J. Biol. Chem. 272, 7003-7012. - PubMed
- Dahms, N.M., Lobel, P., and Kornfeld, S. (1989). Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Biol. Chem. 264, 12115-12118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous