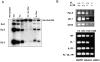Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis - PubMed (original) (raw)
. 2003 Nov 17;198(10):1495-506.
doi: 10.1084/jem.20031152. Epub 2003 Nov 10.
Cord Brakebusch, Inga-Lill Martensson, Marcus Svensson, William W Agace, Mikael Sigvardsson, Natalija Buza-Vidas, David Bryder, Corrado M Cilio, Henrik Ahlenius, Eugene Maraskovsky, Jacques J Peschon, Sten Eirik W Jacobsen
Affiliations
- PMID: 14610045
- PMCID: PMC2194121
- DOI: 10.1084/jem.20031152
Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis
Ewa Sitnicka et al. J Exp Med. 2003.
Abstract
Extensive studies of mice deficient in one or several cytokine receptors have failed to support an indispensable role of cytokines in development of multiple blood cell lineages. Whereas B1 B cells and Igs are sustained at normal levels throughout life of mice deficient in IL-7, IL-7Ralpha, common cytokine receptor gamma chain, or flt3 ligand (FL), we report here that adult mice double deficient in IL-7Ralpha and FL completely lack visible LNs, conventional IgM+ B cells, IgA+ plasma cells, and B1 cells, and consequently produce no Igs. All stages of committed B cell progenitors are undetectable in FL-/- x IL-7Ralpha-/- BM that also lacks expression of the B cell commitment factor Pax5 and its direct target genes. Furthermore, in contrast to IL-7Ralpha-/- mice, FL-/- x IL-7Ralpha-/- mice also lack mature B cells and detectable committed B cell progenitors during fetal development. Thus, signaling through the cytokine tyrosine kinase receptor flt3 and IL-7Ralpha are indispensable for fetal and adult B cell development.
Figures
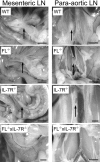
Figure 1.
Mice double deficient in flt3 ligand and IL-7Rα expression show impairment in the development of lymphoid tissue. Photographs of mesenteric and para-aortic LNs taken with 2.2 to 3.45 magnification; arrows point to location of LNs. Bar, 2.2 cm.
Figure 2.
Adult mice double deficient in Flt3 ligand and IL-7Rα expression lack B cells in the PB, spleen, and BM. PB, spleen, and BM cells from 8–12-wk-old mice were stained with mAbs against B220, IgM, CD19, and NK1.1 (as described in Materials and Methods). (A) Plots of B220 and IgM expression in WT, FL-deficient, IL-7Rα–deficient and FL−/− × IL-7Rα−/− double deficient mice, respectively. Numbers represent mean values from 6–12 mice. (B) Total number of B220+ cells coexpressing IgM, B220+ cells expressing CD19 (C), B220+ cells (D), and B220+ cells expressing NK1.1 (E). BM cellularity represents cell counts from both tibiae and femora, whereas total number of white blood cells in PB was calculated per 1 ml. Data in B–E are expressed as mean values (SD) from 6–12 age-matched mice in each group. *Statistically significant differences (P < 0.01) between IL-7Rα−/− and FL−/− × IL-7Rα−/− mice. 0, no B220+IgM+ or B220+CD19+ cells were detected in FL−/− × IL-7Rα−/− mice.
Figure 3.
Mice double deficient in Flt3 ligand and IL-7Rα expression have reduced numbers of IgA+ plasma cells and B1 cells. (A). Cells from lamina propria of 8–12-wk-old WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice were stained with mAbs against IgA (as described in Materials and Methods). 0, no IgA+ cells were detected in FL−/− × IL-7Rα−/− mice. (B) Cells from peritoneal lavage of 8–12-wk-old WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice were stained with mAbs against B220 and CD5 (described in Materials and Methods). All data are expressed as mean values (SD) from three to six mice. *Indicates statistically significant differences (P < 0.01) between IL-7Rα−/− and FL−/− × IL-7Rα−/− mice. 0, no B220+CD5+ cells were detected in FL−/− × IL-7Rα−/− mice.
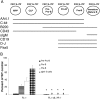
Figure 4.
Mice double deficient in flt3 ligand and IL-7Rα expression show dramatic reductions in early and late B cell progenitors in adult BM. (A) Schematic illustration of cell surface phenotype, B cell gene expression, and D-J rearrangement during normal B cell development. Also shown are expression patterns for flt3 and IL-7R. MPP, multipotent progenitor, Lin−Sca-1+c-kit+flt3+ IL-7R− (63). CLP, common lymphoid progenitor; sIgM, surface IgM. (B) BM cells from WT, FL−/−, and FL−/− × IL-7Rα−/− mice were investigated for the presence of B cell progenitors as described previously (29, 50); pre-pro-B cells (B220+CD43+AA4.1+CD19−), pro-B cells (B220+CD43+AA4.1+CD19+), pre-B cells (B220+CD43−CD19+), and immature/mature B cells (B220+IgM+). Results are mean values (SD) from six to twelve mice for each group and are expressed as percentages of WT controls (no significant differences in BM cellularity between different genotypes). *Indicates statistically significant differences (P < 0.01) between FL−/− and FL−/− × IL-7Rα−/− mice. 0, no pro-B, pre-B, or B220+IgM+ cells were detected in FL−/− × IL-7Rα−/− mice.
Figure 5.
Absence of expression of Pax5 and Pax5 target genes in BM of adult FL−/− × IL-7Rα−/− mice. (A) DNA was isolated from BM of WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice and D-J rearrangement assessed by PCR (described in Materials and Methods). Representative results from two out of seven analyzed mice. (B) RNA was isolated from BM of WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice (described in Material and Methods) and expression of Pax5, mb-1, and CD19 (top) or HGPRT (bottom) analyzed by RT-PCR. Data are from one of three experiments with similar results.
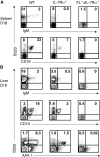
Figure 6.
Lack of detectable mature B cells and B cell progenitors during fetal development of mice double deficient in flt3 ligand and IL-7Rα expression. Spleen and liver cells isolated from embryos at day 17–18 of gestation were stained with mAbs against B220, CD19, IgM, and AA4.1 (Materials and Methods). (A) Plots of B220, CD19, and IgM expression in the spleens of WT, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice. Numbers represent mean values from three to eight mice. (B) Plots of B220, CD19, and IgM expression in the livers of WT, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice. Numbers in A and B represent mean values (percentage of total cells in lymphoid gate) from three to eight mice.
Similar articles
- Origins of peripheral B cells in IL-7 receptor-deficient mice.
Hesslein DG, Yang SY, Schatz DG. Hesslein DG, et al. Mol Immunol. 2006 Feb;43(4):326-34. doi: 10.1016/j.molimm.2005.02.010. Mol Immunol. 2006. PMID: 16310046 - Expression analysis and characterization of alternatively spliced transcripts of human IL-7Ralpha chain encoding two truncated receptor proteins in relapsed childhood all.
Korte A, Köchling J, Badiali L, Eckert C, Andreae J, Geilen W, Kebelmann-Betzing C, Taube T, Wu S, Henze G, Seeger K. Korte A, et al. Cytokine. 2000 Nov;12(11):1597-608. doi: 10.1006/cyto.2000.0777. Cytokine. 2000. PMID: 11052810 - Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency.
Puel A, Ziegler SF, Buckley RH, Leonard WJ. Puel A, et al. Nat Genet. 1998 Dec;20(4):394-7. doi: 10.1038/3877. Nat Genet. 1998. PMID: 9843216 - The biological role and potential therapeutic application of interleukin 7.
Krawczenko A, Kieda C, Duś D. Krawczenko A, et al. Arch Immunol Ther Exp (Warsz). 2005 Nov-Dec;53(6):518-25. Arch Immunol Ther Exp (Warsz). 2005. PMID: 16407784 Review. - IL-7: a key regulator of B lymphopoiesis.
Milne CD, Paige CJ. Milne CD, et al. Semin Immunol. 2006 Feb;18(1):20-30. doi: 10.1016/j.smim.2005.10.003. Epub 2005 Nov 21. Semin Immunol. 2006. PMID: 16303314 Review.
Cited by
- Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning.
Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Bhattacharya D, et al. J Exp Med. 2006 Jan 23;203(1):73-85. doi: 10.1084/jem.20051714. Epub 2005 Dec 27. J Exp Med. 2006. PMID: 16380511 Free PMC article. - IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF.
Kikuchi K, Lai AY, Hsu CL, Kondo M. Kikuchi K, et al. J Exp Med. 2005 Apr 18;201(8):1197-203. doi: 10.1084/jem.20050158. J Exp Med. 2005. PMID: 15837809 Free PMC article. - Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5.
Roessler S, Györy I, Imhof S, Spivakov M, Williams RR, Busslinger M, Fisher AG, Grosschedl R. Roessler S, et al. Mol Cell Biol. 2007 Jan;27(2):579-94. doi: 10.1128/MCB.01192-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101802 Free PMC article. - STAT3 positively regulates an early step in B-cell development.
Chou WC, Levy DE, Lee CK. Chou WC, et al. Blood. 2006 Nov 1;108(9):3005-11. doi: 10.1182/blood-2006-05-024430. Epub 2006 Jul 6. Blood. 2006. PMID: 16825489 Free PMC article. - Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment.
Vosshenrich CA, Cumano A, Müller W, Di Santo JP, Vieira P. Vosshenrich CA, et al. Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11070-5. doi: 10.1073/pnas.0402919101. Epub 2004 Jul 19. Proc Natl Acad Sci U S A. 2004. PMID: 15263090 Free PMC article.
References
- Morrison, S.J., N. Uchida, and I.L. Weissman. 1995. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11:35–71. - PubMed
- Ogawa, M. 1993. Differentiation and proliferation of hematopoietic stem cells. Blood. 81:2844–2853. - PubMed
- Metcalf, D. 1993. Hematopoietic regulators: redundancy or subtlety? Blood. 82:3515–3523. - PubMed
- Zhu, J., and S.G. Emerson. 2002. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 21:3295–3313. - PubMed
- Lotem, J., and L. Sachs. 2002. Cytokine control of developmental programs in normal hematopoiesis and leukemia. Oncogene. 21:3284–3294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous