Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice - PubMed (original) (raw)
Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice
Laura M Bohn et al. J Neurosci. 2003.
Abstract
The reinforcing and psychomotor effects of morphine involve opiate stimulation of the dopaminergic system via activation of mu-opioid receptors (muOR). Both mu-opioid and dopamine receptors are members of the G-protein-coupled receptor (GPCR) family of proteins. GPCRs are known to undergo desensitization involving phosphorylation of the receptor and the subsequent binding of beta(arrestins), which prevents further receptor-G-protein coupling. Mice lacking beta(arrestin)-2 (beta(arr2)) display enhanced sensitivity to morphine in tests of pain perception attributable to impaired desensitization of muOR. However, whether abrogating muOR desensitization affects the reinforcing and psychomotor properties of morphine has remained unexplored. In the present study, we examined this question by assessing the effects of morphine and cocaine on locomotor activity, behavioral sensitization, conditioned place preference, and striatal dopamine release in beta(arr2) knock-out (beta(arr2)-KO) mice and their wild-type (WT) controls. Cocaine treatment resulted in very similar neurochemical and behavioral responses between the genotypes. However, in the beta(arr2)-KO mice, morphine induced more pronounced increases in striatal extracellular dopamine than in WT mice. Moreover, the rewarding properties of morphine in the conditioned place preference test were greater in the beta(arr2)-KO mice when compared with the WT mice. Thus, beta(arr2) appears to play a more important role in the dopaminergic effects mediated by morphine than those induced by cocaine.
Figures
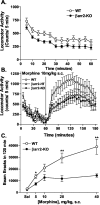
Figure 1.
Locomotor activity after acute morphine treatment. A, Basal locomotor activity in WT and βarr2-KO mice during a 1 hr habituation period (for time, F(11,1022) = 32.89, p < 0.0001; for genotype, F(1,93) = 8.26, p < 0.005; n = 46-49). B, Effects of an acute dose of morphine. Morphine (10 mg/kg, s.c.) was administered to wild-type (n = 8), heterozygotes (n = 8), and βarr2-KO (n = 8) mice after a 1 hr habituation period. Locomotor activity was assessed as the number of infrared beam breaks in 5 min intervals. The values were averaged across mice, and the means ± SEM are shown here. Morphine-induced locomotor activity in all three groups, yet the genotypes did not differ significantly at this dose (for genotype, F(23,483) = 27.94, p < 0.0001; for dose, F(2,21) = 2.62, p = 0.0965). C, Morphine dose-response curve for locomotor activity. The number of beam breaks was summed over the 120 min test period, and the mean ± SEM after morphine or saline (10 μl/g, s.c.) administration is presented. The sum of the beam breaks in B is included in this graph, representing the 10 mg/kg dose. Overall, morphine produced greater increases in locomotor activity in WT mice than in the βarr2-KO mice (for genotype, F(1,95) = 70.22, p < 0.001; for dose, F(4,95) = 36.07, p<0.0001; n = 8-16). Bonferroni post hoc analysis revealed significant differences between the genotypes at the doses of 5, 20, and 40 mg/kg subcutaneous morphine (WT vs KO; *p < 0.05; **p < 0.001).
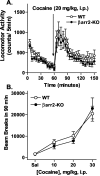
Figure 2.
Locomotor activity after acute cocaine treatment. A, Cocaine (20 mg/kg, i.p.) was administered to WT (n = 15) and βarr2-KO (n = 12) mice after a 1 hr habituation period. Locomotor activity was assessed as described in Figure 1. Although cocaine produced marked increases in locomotor activity in both genotypes, WT mice displayed slightly higher activity than the βarr2-KO mice after morphine (for time, F(17,391) = 12.40, p < 0.0001; for genotype, F(1,23) = 0.96, p = 0.3371). B, Dose-response curve for cocaine-induced locomotor activity. Data represent the sum of the activity after each dose over a 90 min period. There were no significant differences between the genotypes (F(1,62) = 0.08; p = 0.7723; n = 7-15).
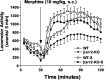
Figure 3.
Locomotor sensitization to morphine. Locomotor activity was initially assessed in WT and βarr2-KO mice during a 30 min habituation period and for 90 min after an acute dose of morphine (10 mg/kg, s.c.). After this observation, mice received one daily dose of morphine (10 mg/kg, s.c.) for 6 additional days in their home cage. After 1 d of rest, the mice were then challenged again with the same dose of morphine (10 mg/kg, s.c.), and locomotor activity was assessed (WT-S, βarr2-KO-S). Locomotor activity was increased significantly after this regimen in both WT and βarr2-KO mice, indicating that sensitization to morphine developed in both genotypes (for sensitization, F(1,36) = 5.68, p < 0.05; for genotype, F(1,36) = 3.78, p = 0.06; n = 10 of each genotype.)
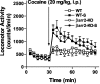
Figure 4.
Locomotor sensitization to cocaine. Locomotor activity was initially assessed in WT and βarr2-KO mice during a 30 min habituation period and for 90 min after an acute dose of cocaine (20 mg/kg, i.p.). Mice then received one daily dose of cocaine (20 mg/kg, i.p.) for 4 additional days in their home cage. After 1 d of rest, the mice were then challenged again with the same dose of cocaine (20 mg/kg, i.p.), and locomotor activity was again assessed (WT-S, βarr2-KO-S). Data represent the sum of the number of beam breaks recorded during 60 min after drug administration. Both groups of mice became sensitized to cocaine to a similar extent (for time, F(1,39) = 7.67, p < 0.01; for genotype, F(1,39) = 0.00, p = 0.9474; n = 7-14.)
Figure 5.
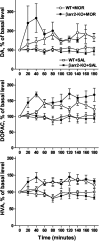
Invivo microdialysis after acute morphine. The effects of morphine (10 mg/kg, s.c.) and saline (10 μl/g, s.c.) on extracellular DA, DOPAC, and HVA levels in the striatum of freely moving mice as measured using in vivo microdialysis. Data are presented as the percentage of the average level of dopamine or metabolite measured in at least three samples collected before the drug administration. Analysis of area under curve values for 3 hr after administration (data not shown) revealed a significant effect of morphine on extracellular dopamine and metabolites in both genotypes: WT+Sal vs WT+Mor, dopamine, DOPAC, and HVA, p < 0.01; βarr2-KO+Sal vs βarr2-KO+Mor, dopamine, DOPAC, and HVA, _p_ < 0.01; Mann-Whitney _U_ test (two-tailed). Analysis of the 1 hr period after morphine administration (data not shown) revealed that βarr2-KO mice produced a greater accumulation of dopamine and metabolites: WT (_n_ = 9) vs βarr2-KO (_n_ = 6) after morphine, DA, _p_ < 0.05; DOPAC and HVA, _p_ < 0.01; Mann-Whitney _U_ test (two-tailed). Similar analysis of saline effects (1 or 3 hr period) revealed no significant differences between the genotypes (WT, _n_ = 5; βarr2-KO, _n_ = 5; _p_ > 0.05; two-tailed Mann-Whitney U test).
Figure 6.
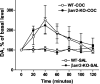
In vivo microdialysis after acute cocaine. The effects of cocaine (20 mg/kg, i.p.) and saline (10 μl/g, i.p.) on extracellular DA levels in the striatum of freely moving mice as measured using in vivo microdialysis. Data are presented as the percentage of the average level of dopamine measured in at least three samples collected before the drug administration. Analysis of area under curve values for 2 hr after cocaine treatment (data not shown) revealed a significant increase in DA in both genotypes compared with the saline treatment of the respective genotype (p < 0.01; two-tailed Mann-Whitney _U_ test). No significant differences were observed between genotypes after saline (WT, _n_ = 5; βarr2-KO, _n_ = 5) or cocaine (WT, _n_ = 5; KO, _n_ = 4; _p_ > 0.05; two-tailed Mann-Whitney U test).
Figure 7.
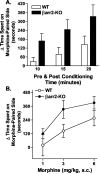
Morphine-induced conditioned place preference. Mice were assessed for the time spent in either the black or white compartment on the preconditioning day. Drug was alternatively paired with either side for both groups of mice as described in Materials and Methods. After the 6 d conditioning regimen (drug on days 1, 3, and 5; saline on days 2, 4, and 6), the time spent in each compartment was assessed in the absence of drug or saline (postconditioning). A, Data are shown as the difference in the time spent in the drug-paired (morphine, 3 mg/kg, s.c.) compartment on the postconditioning day and the preconditioning day when the data are analyzed at 10, 15, or 20 min preconditioning and postconditioning times (mean ± SEM). The βarr2-KO mice spent more time in the drug-paired side than the WT mice when compared over all times (for genotype, F(1,11) = 5.53, p < 0.05; for time, F(2,22) = 15.20, p < 0.0001). B, Dose-response curve at 20 min preconditioning and postconditioning times. Morphine produced a dose-dependent increase in preference for the morphine-paired compartment in both genotypes; however, when comparing genotypes, the effect was significantly greater in the βarr2-KO mice compared with their WT littermates (for genotype, F(1,41) = 6.31, p < 0.05; for dose, F(2,41) = 7.05, p < 0.01; n = 9 per group).
Figure 8.
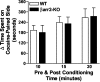
Cocaine-induced conditioned place preference. Conditioning was performed as described in Figure 7_A_ with cocaine (10 mg/kg, i.p.). Data are shown as the difference in the time spent in the drug-paired compartment on the postconditioning day and the preconditioning day when the preconditioning and postconditioning times were set at 10, 15, or 20 min (mean ± SEM). Cocaine (10 mg/kg, i.p.) produced preference for the cocaine-paired compartment in both genotypes that did not differ under any of the conditions (for genotype, F(1,16) = 0.05, p = 0.8179; for time, F(2,32) = 5.95, p < 0.01; n = 9-10 per group).
Similar articles
- Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice.
Bohn LM, Lefkowitz RJ, Caron MG. Bohn LM, et al. J Neurosci. 2002 Dec 1;22(23):10494-500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. J Neurosci. 2002. PMID: 12451149 Free PMC article. - A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice.
Urs NM, Daigle TL, Caron MG. Urs NM, et al. Neuropsychopharmacology. 2011 Feb;36(3):551-8. doi: 10.1038/npp.2010.186. Epub 2010 Oct 27. Neuropsychopharmacology. 2011. PMID: 20980993 Free PMC article. - Evidence that behavioral phenotypes of morphine in β-arr2-/- mice are due to the unmasking of JNK signaling.
Mittal N, Tan M, Egbuta O, Desai N, Crawford C, Xie CW, Evans C, Walwyn W. Mittal N, et al. Neuropsychopharmacology. 2012 Jul;37(8):1953-62. doi: 10.1038/npp.2012.42. Epub 2012 Apr 11. Neuropsychopharmacology. 2012. PMID: 22491351 Free PMC article. - Opioid tolerance: is there a dialogue between glutamate and beta-arrestin?
Chiao YC, Wong CS. Chiao YC, et al. Acta Anaesthesiol Taiwan. 2004 Jun;42(2):93-101. Acta Anaesthesiol Taiwan. 2004. PMID: 15346705 Review. - The physiological relevance of functional selectivity in dopamine signalling.
Urs NM, Caron MG. Urs NM, et al. Int J Obes Suppl. 2014 Jul;4(Suppl 1):S5-8. doi: 10.1038/ijosup.2014.3. Epub 2014 Jul 8. Int J Obes Suppl. 2014. PMID: 27152166 Free PMC article. Review.
Cited by
- Toward Directing Opioid Receptor Signaling to Refine Opioid Therapeutics.
Grim TW, Acevedo-Canabal A, Bohn LM. Grim TW, et al. Biol Psychiatry. 2020 Jan 1;87(1):15-21. doi: 10.1016/j.biopsych.2019.10.020. Epub 2019 Oct 31. Biol Psychiatry. 2020. PMID: 31806082 Free PMC article. Review. - The Utilization of Mu-Opioid Receptor Biased Agonists: Oliceridine, an Opioid Analgesic with Reduced Adverse Effects.
Urits I, Viswanath O, Orhurhu V, Gress K, Charipova K, Kaye AD, Ngo A. Urits I, et al. Curr Pain Headache Rep. 2019 Mar 18;23(5):31. doi: 10.1007/s11916-019-0773-1. Curr Pain Headache Rep. 2019. PMID: 30880365 Review. - A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework.
Hoare SRJ, Tewson PH, Quinn AM, Hughes TE. Hoare SRJ, et al. Sci Rep. 2020 Feb 4;10(1):1766. doi: 10.1038/s41598-020-58421-9. Sci Rep. 2020. PMID: 32019973 Free PMC article. - Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission.
Guzman MS, De Jaeger X, Raulic S, Souza IA, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, Prado VF, Prado MA. Guzman MS, et al. PLoS Biol. 2011 Nov;9(11):e1001194. doi: 10.1371/journal.pbio.1001194. Epub 2011 Nov 8. PLoS Biol. 2011. PMID: 22087075 Free PMC article. - Loss of Astrocytic µ Opioid Receptors Exacerbates Aversion Associated with Morphine Withdrawal in Mice: Role of Mitochondrial Respiration.
Murlanova K, Jouroukhin Y, Novototskaya-Vlasova K, Huseynov S, Pletnikova O, Morales MJ, Guan Y, Kamiya A, Bergles DE, Dietz DM, Pletnikov MV. Murlanova K, et al. Cells. 2023 May 17;12(10):1412. doi: 10.3390/cells12101412. Cells. 2023. PMID: 37408246 Free PMC article.
References
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT ( 1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495-2498. - PubMed
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG ( 2000) Muopioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720-723. - PubMed
- Brase DA, Loh HH, Way EL ( 1977) Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther 201: 368-374. - PubMed
- Chefer VI, Shippenberg TS ( 2002) Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience 112: 907-919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials