Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts - PubMed (original) (raw)
Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts
Dmitry E Shvartsman et al. J Cell Biol. 2003.
Abstract
Lipid rafts play important roles in cellular functions through concentrating or sequestering membrane proteins. This requires proteins to differ in the stability of their interactions with lipid rafts. However, knowledge of the dynamics of membrane protein-raft interactions is lacking. We employed FRAP to measure in live cells the lateral diffusion of influenza hemagglutinin (HA) proteins that differ in raft association. This approach can detect weak interactions with rafts not detectable by biochemical methods. Wild-type (wt) HA and glycosylphosphatidylinositol (GPI)-anchored HA (BHA-PI) diffused slower than a nonraft HA mutant, but became equal to the latter after cholesterol depletion. When antigenically distinct BHA-PI and wt HA were coexpressed, aggregation of BHA-PI into immobile patches reduced wt HA diffusion rate, suggesting transient interactions with BHA-PI raft patches. Conversely, patching wt HA reduced the mobile fraction of BHA-PI, indicating stable interactions with wt HA patches. Thus, the anchoring mode determines protein-raft interaction dynamics. GPI-anchored and transmembrane proteins can share the same rafts, and different proteins can interact stably or transiently with the same raft domains.
Figures
Figure 1.
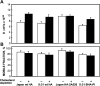
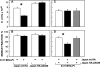
Triton insolubility of HA mutants. Experiments were conducted on Fugene 6–transfected COS-7 cells expressing Japan wt HA or Japan HA-2A520. Similar results (not depicted) were obtained on CV-1 cells infected with recombinant SV40 virus vectors. 24 h after transfection, the cells were subjected to cholesterol depletion as described under the Materials and methods. The cells were then labeled with [35S]methionine and cysteine, chased 100 min in serum-free DME, and lysed 20 min on ice in 1 ml lysis buffer containing 1% Triton X-100. The lysates were separated into soluble and insoluble fractions by centrifugation, and these were immunoprecipitated with rabbit α-Japan HA antibody and analyzed by PAGE and phosphorimaging. Bars show the average ± SD of three to five replicates.
Figure 2.
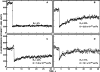
Representative FRAP curves measuring the lateral diffusion of Japan wt HA. CV-1 cells were infected with recombinant SV40 virions encoding Japan wt HA. After 16–18 h, the cells were either subjected to cholesterol depletion for 18–20 h, or left untreated for the same period. 36 h after infection, cell surface HAs were labeled with α-Japan TRITC-Fab' (100 μg/ml, 45 min, 4°C), and FRAP studies were conducted at 22°C. Solid lines are the best fit to the lateral diffusion equation using nonlinear regression (Fire et al., 1995). The lateral diffusion coefficients (D) and mobile fraction values (R f) for each curve are shown. (A) Japan wt HA diffusion in an untreated cell. (B) Japan wt HA diffusion in a cholesterol-depleted cell.
Figure 3.
Cholesterol depletion elevates the lateral diffusion rates of DRM-associated HAs to the level of the nonraft HA-2A520. The experiments were performed as in Fig. 2, on CV-1 cells infected with recombinant SV40 virions (36 h after infection) or transiently transfected with the relevant SV40 expression vectors encoding each HA mutant (48 h after transfection). Analogous experiments were conducted on COS-7 cells transiently transfected with the same vectors (24 h after transfection). In all cases, these incubation periods include 18–20-h cholesterol depletion treatment where applicable. Similar results were obtained on both cell types. Cell surface HAs were labeled with monovalent Fab' (100 μg/ml α-Japan TRITC-Fab' for Japan HAs, or 50 μg/ml α-X:31 rabbit Fab' followed by 50 μg/ml GαR TRITC-Fab' for X:31 HAs). Each bar is the mean ± SEM of 30–40 measurements. (A) D values. D of Japan HA-2A520 on untreated cells was significantly higher than D of wt HA (Japan or X:31) or of X:31 BHA-PI (P < 0.001). The effects of cholesterol depletion on the _D_ values of the wt HAs (P < 0.001) and X:31 BHA-PI (P < 0.01) were significant; _D_ of Japan HA-2A520 was not significantly affected by this treatment (P > 0.25). (B) Mobile fraction (R f) values. There were no significant differences between the R f values of Japan wt HA and Japan HA-2A520 (P > 0.1), or between X:31 wt HA and X:31 BHA-PI (P > 0.25). The R f values were slightly reduced after cholesterol depletion, but this effect was not significant in all cases (P > 0.05) and was observed also for the Japan HA-2A520 mutant.
Figure 4.
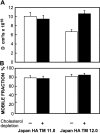
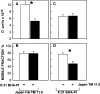
Cholesterol-sensitive differences between the lateral diffusion rates of Japan HA TM 12.0 and Japan HA TM 11.0 mutants. The experiments were performed as in Fig. 2, on COS-7 cells transiently transfected with the relevant SV40 expression vectors encoding each HA mutant (24 h after transfection). This period includes 18–20-h cholesterol depletion treatment where applicable. Each bar is the mean ± SEM of 30–40 measurements. (A) D values. D of TM 11.0 on untreated cells was significantly higher than D of TM 12.0 (P < 0.001). The increase in _D_ of TM 12.0 after cholesterol depletion was also significant (P < 0.001), while _D_ of TM 11.0 was not affected (P > 0.25). (B) Mobile fraction (R f) values. The differences between R f of the two mutants, with or without cholesterol depletion, were not significant (P > 0.20).
Figure 5.
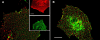
Co-patching between pairs of Japan and X:31 HA mutants. CV-1 cells were transfected transiently (see Materials and methods) by pairs of expression vectors encoding various HA mutants. Experiments were conducted 48 h after transfection. Where applicable, the cells were subjected to cholesterol depletion for the last 18–20 h after transfection. Live cells were consecutively labeled at 4°C by a series of antibodies (45 min each incubation) to mediate patching and fluorescent labeling (see Materials and methods), resulting in Japan HA mutants labeled by FITC (green) and X:31 HA mutants labeled by Alexa®594 (red). Bars, 20 μm. Similar results were obtained when the incubation periods were extended to 2 h (4 h total), or when the incubation temperature was increased to 18°C. (A) Co-patching between Japan wt HA and X:31 wt HA. The insets demonstrate the specificity of the labeling and patching protocol. Cells singly transfected with one HA type display exclusively red (X:31 wt HA, top inset) or green (Japan wt HA, bottom inset) fluorescence, although they were labeled with antibody mixtures for HAs from both strains. (B) Co-patching between X:31 BHA-PI and Japan wt HA. (C) Quantification of co-patching. Superimposed red and green images were analyzed by counting the numbers of green (G), red (R), and overlapping (yellow, Y) patches as described under the Materials and methods, counting patches in flat membrane regions (10 × 10 μm2) on 20–25 cells in each case. The percent co-patching (% of one protein in mutual patches with the coexpressed HA protein) is given by 100 × (Y/[Y + G]) and by 100 × (Y/[Y + R]) for the green- and red-labeled HAs, respectively. As the results were similar (within 2%) for green and red labeling, the average value is shown for each pair. The bars are mean ± SEM. Except for the pair X:31 BHA-PI/Japan wt HA, the co-patching levels of the various pairs on untreated cells were not significantly different from the control pairs of X:31 wt HA or X:31 BHA-PI with Japan HA-2A520 (P > 0.25). The % co-patching of BHA-PI/Japan wt HA was significantly higher (P < 0.001) and was significantly reduced by cholesterol depletion (P < 0.001).
Figure 5.
Co-patching between pairs of Japan and X:31 HA mutants. CV-1 cells were transfected transiently (see Materials and methods) by pairs of expression vectors encoding various HA mutants. Experiments were conducted 48 h after transfection. Where applicable, the cells were subjected to cholesterol depletion for the last 18–20 h after transfection. Live cells were consecutively labeled at 4°C by a series of antibodies (45 min each incubation) to mediate patching and fluorescent labeling (see Materials and methods), resulting in Japan HA mutants labeled by FITC (green) and X:31 HA mutants labeled by Alexa®594 (red). Bars, 20 μm. Similar results were obtained when the incubation periods were extended to 2 h (4 h total), or when the incubation temperature was increased to 18°C. (A) Co-patching between Japan wt HA and X:31 wt HA. The insets demonstrate the specificity of the labeling and patching protocol. Cells singly transfected with one HA type display exclusively red (X:31 wt HA, top inset) or green (Japan wt HA, bottom inset) fluorescence, although they were labeled with antibody mixtures for HAs from both strains. (B) Co-patching between X:31 BHA-PI and Japan wt HA. (C) Quantification of co-patching. Superimposed red and green images were analyzed by counting the numbers of green (G), red (R), and overlapping (yellow, Y) patches as described under the Materials and methods, counting patches in flat membrane regions (10 × 10 μm2) on 20–25 cells in each case. The percent co-patching (% of one protein in mutual patches with the coexpressed HA protein) is given by 100 × (Y/[Y + G]) and by 100 × (Y/[Y + R]) for the green- and red-labeled HAs, respectively. As the results were similar (within 2%) for green and red labeling, the average value is shown for each pair. The bars are mean ± SEM. Except for the pair X:31 BHA-PI/Japan wt HA, the co-patching levels of the various pairs on untreated cells were not significantly different from the control pairs of X:31 wt HA or X:31 BHA-PI with Japan HA-2A520 (P > 0.25). The % co-patching of BHA-PI/Japan wt HA was significantly higher (P < 0.001) and was significantly reduced by cholesterol depletion (P < 0.001).
Figure 6.
Representative FRAP curves measuring the effects of immobilizing one HA protein on the lateral diffusion of another. Cells were cotransfected with pairs of antigenically distinct HA proteins. After 48 h (CV-1 cells) or 24 h (COS-7 cells), one type of HA was cross-linked by IgGs (30 μg/ml HC3 mouse α-X:31 HA or Fc125 mouse α-Japan HA, followed by 30 μg/ml GαM FITC-IgG), while the coexpressed HA type was labeled by monovalent Fab' (100 μg/ml α-Japan rabbit TRITC-Fab', or 50 μg/ml α-X:31 rabbit Fab' followed by 50 μg/ml GαR TRITC-Fab'). FRAP experiments were conducted at 22°C as in Fig. 2, identifying coexpressing cells by their FITC fluorescence. (A) Lateral diffusion of IgG–cross-linked X:31 BHA-PI. Only the R f value is shown, as the recovery was too low to allow determination of D. Similar immobilization was measured for IgG–cross-linked Japan wt HA or Japan HA-2A520. (B) Lateral diffusion of Japan wt HA in the presence of IgG–cross-linked X:31 BHA-PI. (C) Effect of cross-linking Japan wt HA on the lateral diffusion of X:31 BHA-PI. (D) Lack of effect of cross-linking X:31 BHA-PI on the lateral diffusion of Japan HA-2A520.
Figure 7.
Cross-linking of one HA in a pair of coexpressed HA proteins has differential effects on the lateral diffusion of wt HA and BHA-PI. Experiments were performed as in Fig. 6. Bars are mean ± SEM of 20–30 measurements. Asterisks indicate significant differences from the uncross-linked control (P < 0.001, t test). (A and B) Lateral diffusion of Japan wt HA and Japan HA-2A520 in the presence (+) or absence (−) of coexpressed, IgG–cross-linked X:31 BHA-PI. Control experiments in which coexpressed X:31 BHA-PI was labeled with FITC-Fab' to avoid cross-linking and immobilization yielded results similar to the (−) control. (C and D) Lateral diffusion of X:31 BHA-PI in the presence (+) or absence (−) of coexpressed, IgG–cross-linked Japan wt HA or Japan HA-2A520. Labeling the coexpressed Japan HAs with FITC-Fab' to avoid cross-linking and immobilization yielded results similar to singly expressed BHA-PI.
Figure 8.
Cross-linking/FRAP studies demonstrate transient association of HA TM 11.0 with rafts. Experiments were conducted as in Fig. 7. Bars are mean ± SEM of 25 measurements. Significant differences (P < 0.001) are indicated by asterisks. (A and B) Lateral diffusion of Japan HA TM 11.0 with (+) or without (−) IgG–cross-linked X:31 BHA-PI. (C and D) Lateral diffusion of X:31 BHA-PI in the presence (+) or absence (−) of IgG–cross-linked Japan HA TM 11.0.
Similar articles
- Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion.
Takeda M, Leser GP, Russell CJ, Lamb RA. Takeda M, et al. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14610-7. doi: 10.1073/pnas.2235620100. Epub 2003 Oct 15. Proc Natl Acad Sci U S A. 2003. PMID: 14561897 Free PMC article. - Influenza A virus hemagglutinin and neuraminidase mutually accelerate their apical targeting through clustering of lipid rafts.
Ohkura T, Momose F, Ichikawa R, Takeuchi K, Morikawa Y. Ohkura T, et al. J Virol. 2014 Sep 1;88(17):10039-55. doi: 10.1128/JVI.00586-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965459 Free PMC article. - Clustering of raft-associated proteins in the external membrane leaflet modulates internal leaflet H-ras diffusion and signaling.
Eisenberg S, Shvartsman DE, Ehrlich M, Henis YI. Eisenberg S, et al. Mol Cell Biol. 2006 Oct;26(19):7190-200. doi: 10.1128/MCB.01059-06. Mol Cell Biol. 2006. PMID: 16980621 Free PMC article. - [Lipid raft and influenza virus--viral glycoproteins on a raft].
Takeda M. Takeda M. Uirusu. 2004 Jun;54(1):9-15. doi: 10.2222/jsv.54.9. Uirusu. 2004. PMID: 15449899 Review. Japanese. - GPI-anchored proteins and lipid rafts.
Sangiorgio V, Pitto M, Palestini P, Masserini M. Sangiorgio V, et al. Ital J Biochem. 2004 Jul;53(2):98-111. Ital J Biochem. 2004. PMID: 15646015 Review.
Cited by
- Membrane microdomains emergence through non-homogeneous diffusion.
Soula HA, Coulon A, Beslon G. Soula HA, et al. BMC Biophys. 2012 Apr 30;5:6. doi: 10.1186/2046-1682-5-6. BMC Biophys. 2012. PMID: 22546236 Free PMC article. - Both MHC class II and its GPI-anchored form undergo hop diffusion as observed by single-molecule tracking.
Umemura YM, Vrljic M, Nishimura SY, Fujiwara TK, Suzuki KG, Kusumi A. Umemura YM, et al. Biophys J. 2008 Jul;95(1):435-50. doi: 10.1529/biophysj.107.123018. Epub 2008 Mar 13. Biophys J. 2008. PMID: 18339737 Free PMC article. - Cholesterol depletion suppresses the translational diffusion of class II major histocompatibility complex proteins in the plasma membrane.
Vrljic M, Nishimura SY, Moerner WE, McConnell HM. Vrljic M, et al. Biophys J. 2005 Jan;88(1):334-47. doi: 10.1529/biophysj.104.045989. Epub 2004 Oct 29. Biophys J. 2005. PMID: 15516525 Free PMC article. - A Mycobacterium tuberculosis-derived lipid inhibits membrane fusion by modulating lipid membrane domains.
Hayakawa E, Tokumasu F, Nardone GA, Jin AJ, Hackley VA, Dvorak JA. Hayakawa E, et al. Biophys J. 2007 Dec 1;93(11):4018-30. doi: 10.1529/biophysj.107.104075. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704144 Free PMC article. - Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling.
Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Hartung A, et al. Mol Cell Biol. 2006 Oct;26(20):7791-805. doi: 10.1128/MCB.00022-06. Epub 2006 Aug 21. Mol Cell Biol. 2006. PMID: 16923969 Free PMC article.
References
- Anderson, R.G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. - PubMed
- Brewer, C.B. 1994. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods Cell Biol. 43:233–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous