Avidity modulation activates adhesion under flow and requires cooperativity among adhesion receptors - PubMed (original) (raw)
Avidity modulation activates adhesion under flow and requires cooperativity among adhesion receptors
Na Ni et al. Biophys J. 2003 Dec.
Abstract
An early step in activation of leukocyte adhesion is a release of integrins from cytoskeletal constraints on their diffusion, leading to rearrangement and, consequently, increased avidity. Static adhesion assays using purified ligand as a substrate have demonstrated that very low doses of cytochalasin D disconnect beta2-integrins from their cytoskeletal links, allowing rearrangement and activating adhesion. The adhesion process in blood vessels is poorly simulated by these assays, however, for two reasons: leukocyte adhesion to endothelium 1), occurs in the presence of blood flow and 2), involves the simultaneous interactions of multiple sets of adhesion molecules. We investigated the effect of cytochalasin D, at concentrations that increase integrin diffusion but do not alter leukocyte shape and surface features, on adhesion of leukocytes to endothelial cells under flow. Cytochalasin D increased the number of rolling cells, the number of firmly adherent cells, and the duration of both rolling and firm adhesion. These effects required endothelial cell expression of ICAM-1, the ligand for leukocyte beta2-integrins. The beta2-integrin-ICAM-1 interaction alone was not sufficient, however. Experiments using purified substrates demonstrated that avidity effects on activation of adhesion under flow require functional cooperativity between integrins and other adhesion receptors.
Figures
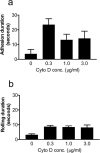
FIGURE 1
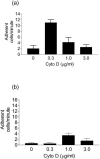
Cytochalasin D activates adhesion under flow in a dose-dependent manner. Nonrolling adhesion (arrest) of WEHI 274.1 cells on cultured primary endothelial cells from both wild-type (a) and ICAM-1 −/− (b) mouse aortas was quantified. At a physiologic shear stress of 2.5 dynes/cm2, adhesion was increased fivefold on wild-type endothelium by low-dose cytochalasin D. On ICAM-1 −/− endothelium, the probability of arrest was lower. Cytochalasin D had no effect on adhesion at 0.3_μ_g/ml, although a small increase was seen at 1.0 _μ_g/ml.
FIGURE 2
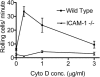
Cytochalasin D activation of rolling requires ICAM-1. Rolling adhesion on both wild-type (squares) and ICAM-1 −/− (triangles) endothelium at a shear stress of 2.5 dynes/cm2 was quantified. Cells were more likely to roll on endothelium that expressed ICAM-1. Moreover, on wild-type endothelium only, rolling was increased up to fourfold by low-dose cytochalasin D, with a dose dependence similar to that for firm adhesion.
FIGURE 3
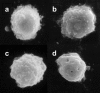
Effect of cytochalasin D on WEHI monocyte morphology. The low doses of cytochalasin D that activate adhesion (b and c) do not cause gross morphological changes, as compared to control (a). Higher doses that inhibit adhesion, however, cause loss of cell surface features (d).
FIGURE 4
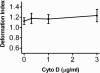
Effect of cytochalasin D on WEHI cell deformability. Cell deformability indices (ratio of cell length to cell width under conditions of shear stress) were calculated for rolling and adherent cells at the shear stress used for adhesion assays (2.5 dynes/cm2) and mean ± SD were plotted as a function of cytochalasin D dose. No significant increase in deformability index was observed at the cytochalasin D concentrations used in this study.
FIGURE 5
Duration of adhesion and rolling. Duration of arrest (a) and rolling (b) were quantified for WEHI monocytes on wild-type endothelium at a shear stress of 2.5 dynes/cm2. Cell trajectories were divided into segments in which the cell was rolling or stopped, as defined in Materials and Methods, to calculate these quantities. The bar graphs represent mean duration (± SE) of these segments for each cytochalasin D concentration. Cytochalasin D increased duration of both types of adhesion.
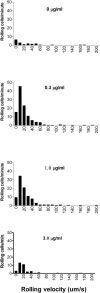
FIGURE 6
Cytochalasin D does not affect rolling velocity. Velocities of WEHI monocytes rolling on wild-type endothelium were quantified, and the distribution of average velocities was expressed as velocity histograms. The difference in bar heights among panels reflects differences in efficiency of cell capture, consistent with Fig. 2. Although cytochalasin D affected the number of rolling cells, the average velocity of those that rolled was not affected.
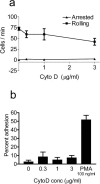
FIGURE 7
Cytochalasin D does not activate rolling or adhesion on purified VCAM-1. (a) To test for an effect of cytochalasin D on _α_4_β_1-integrin avidity, rolling and firm adhesion of WEHI monocytes were quantified at a shear stress of 2.5 dynes/cm2 on a substrate of the _α_4_β_1 ligand VCAM-1. Low dose cytochalasin D was unable to activate either type of adhesion. (b) Adhesion of WEHI monocytes to purified VCAM-1 with 1 min static incubation. WEHI cells were allowed to settle on VCAM-1 for 1 min before being subjected to a shear stress of 2.5 dynes/cm2. Cytochalasin D was unable to increase adhesion in this static assay, whereas PMA-induced adhesion was clearly demonstrable.
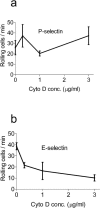
FIGURE 8
Cytochalasin D does not activate rolling on purified selectins. To test whether low-dose cytochalasin D affects avidity of selectin ligands, rolling was quantified on substrates of purified P-selectin (a) and E-selectin (b). Cytochalasin D was unable to activate selectin-mediated rolling adhesions and actually significantly decreased rolling on E-selectin.
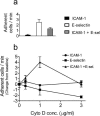
FIGURE 9
Adhesion to a mixture of purified ICAM-1 and E-selectin. WEHI monocytes were perfused at a shear stress of 2.5 dynes/cm2 over substrates of purified ICAM-1, E-selectin, or a mixture of both. (a) Baseline adhesion to purified substrates without cytochalasin D treatment. Note that interaction of flowing cells with ICAM-1 alone is poor. (b) To compare activation of adhesion by cytochalasin D, baseline values are normalized to zero, and changes in adhesion are plotted as a function of cytochalasin D concentration. Although cytochalasin D failed to activate adhesion on either purified ICAM-1 or E-selectin alone, a dose-dependent effect similar to that seen with whole endothelium was observed with both adhesion molecules combined.
Similar articles
- Chemokine stimulation of lymphocyte alpha 4 integrin avidity but not of leukocyte function-associated antigen-1 avidity to endothelial ligands under shear flow requires cholesterol membrane rafts.
Shamri R, Grabovsky V, Feigelson SW, Dwir O, Van Kooyk Y, Alon R. Shamri R, et al. J Biol Chem. 2002 Oct 18;277(42):40027-35. doi: 10.1074/jbc.M206806200. Epub 2002 Aug 5. J Biol Chem. 2002. PMID: 12163503 - Conjugated linoleic acid targets β2 integrin expression to suppress monocyte adhesion.
de Gaetano M, Dempsey E, Marcone S, James WG, Belton O. de Gaetano M, et al. J Immunol. 2013 Oct 15;191(8):4326-36. doi: 10.4049/jimmunol.1300990. Epub 2013 Sep 18. J Immunol. 2013. PMID: 24048900 - Engagement of PSGL-1 enhances beta(2)-integrin-involved adhesion of neutrophils to recombinant ICAM-1.
Wang XG, Cheng YP, Ba XQ. Wang XG, et al. Acta Pharmacol Sin. 2006 May;27(5):617-22. doi: 10.1111/j.1745-7254.2006.00327.x. Acta Pharmacol Sin. 2006. PMID: 16626518 - Rearrangement of integrins in avidity regulation by leukocytes.
Kucik DF. Kucik DF. Immunol Res. 2002;26(1-3):199-206. doi: 10.1385/IR:26:1-3:199. Immunol Res. 2002. PMID: 12403358 Review. - Endothelial cell interactions and integrins.
Malik AB. Malik AB. New Horiz. 1993 Feb;1(1):37-51. New Horiz. 1993. PMID: 7922392 Review.
Cited by
- Integrin Regulated Autoimmune Disorders: Understanding the Role of Mechanical Force in Autoimmunity.
Banerjee S, Nara R, Chakraborty S, Chowdhury D, Haldar S. Banerjee S, et al. Front Cell Dev Biol. 2022 Mar 18;10:852878. doi: 10.3389/fcell.2022.852878. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372360 Free PMC article. Review. - Membrane mobility of beta2 integrins and rolling associated adhesion molecules in resting neutrophils.
Gaborski TR, Clark A Jr, Waugh RE, McGrath JL. Gaborski TR, et al. Biophys J. 2008 Nov 15;95(10):4934-47. doi: 10.1529/biophysj.108.132886. Epub 2008 Aug 8. Biophys J. 2008. PMID: 18689449 Free PMC article. - Alterations in human breast cancer adhesion-motility in response to changes in cell surface glycoproteins displaying alpha-L-fucose moieties.
Yuan K, Kucik D, Singh RK, Listinsky CM, Listinsky JJ, Siegal GP. Yuan K, et al. Int J Oncol. 2008 Apr;32(4):797-807. Int J Oncol. 2008. PMID: 18360707 Free PMC article. - Increased extracellular pressure enhances cancer cell integrin-binding affinity through phosphorylation of beta1-integrin at threonine 788/789.
Craig DH, Gayer CP, Schaubert KL, Wei Y, Li J, Laouar Y, Basson MD. Craig DH, et al. Am J Physiol Cell Physiol. 2009 Jan;296(1):C193-204. doi: 10.1152/ajpcell.00355.2008. Epub 2008 Nov 12. Am J Physiol Cell Physiol. 2009. PMID: 19005162 Free PMC article. - The tumor cell-host organ interface in the early onset of metastatic organ colonisation.
Gassmann P, Haier J. Gassmann P, et al. Clin Exp Metastasis. 2008;25(2):171-81. doi: 10.1007/s10585-007-9130-6. Epub 2007 Dec 5. Clin Exp Metastasis. 2008. PMID: 18058027 Review.
References
- Alon, R., D. A. Hammer, and T. A. Springer. 1995. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. - PubMed
- Bell, G. I. 1978. Models for the specific adhesion of cells to cells. Science. 200:618–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous