Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables - PubMed (original) (raw)
Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables
Agnieszka Kobielak et al. Nat Cell Biol. 2004 Jan.
Abstract
During epithelial sheet formation, linear actin cables assemble at nascent adherens junctions. This process requires alpha-catenin and actin polymerization, although the underlying mechanism is poorly understood. Here, we show that formin-1 interacts with alpha-catenin, localizes to adherens junctions and nucleates unbranched actin filaments. Furthermore, disruption of the alpha-catenin-formin-1 interaction blocks assembly of radial actin cables and perturbs intercellular adhesion. A fusion protein of the beta-catenin-binding domain of alpha-catenin with the actin polymerization domains of formin-1 rescues formation of adherens junctions and associated actin cables in alpha-catenin-null keratinocytes. These findings provide new insight into how alpha-catenin orchestrates actin dynamics during intercellular junction formation.
Figures
Figure 1
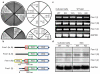
Formin 1 is a putative interacting protein for α-catenin. (a) Full-length α-catenin linked to the Gal4 DNA-binding domain (BD) was used as bait to isolate proteins consisting of the Gal4 activating domain (AD) fused to sequences encoded by mouse skin cDNAs. Note specific interactions between α-catenin and two of its known interacting proteins, β-catenin (β-cat) and plakoglobin (pg), both of which were identified in the screen. Additionally, two clones encoding portions of formin-1 isoforms were identified: formin-1 (IV), a previously identified isoform, and formin-1 (V), a novel isoform. (b) A schematic representation of formin-1 isoforms. Formin-1 (Ia, Ib, II, III and IV) have been reported. Formin-1 (V) was sequenced in its entirety. The sequences encoded by the two interacting clones are underlined, with their corresponding amino-acid residues indicated. FH, formin homology; cc, coiled-coil domain. (c) RT-PCR analysis to detect mRNAs encoding specific formin-1 isoforms in keratinocytes cultured from α-catenin conditional null (knockout) and wild-type mouse skin cultures, and in vivo skin from E13.5, E15.5 and newborn (NB) animals. GAPDH was used as a positive control. Bands specific for isoforms I, IV and V were detected and were of the predicted sizes. If at all present, mRNAs encoding isoforms II and III were expressed at levels below the limits of detection. (d) Northern blot analyses of the mRNA expression patterns of formin-1 isoforms in cultured keratinocytes and whole skin from wild-type and knockout mice.
Figure 2
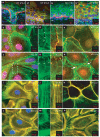
The localization of Formin-1 is dependent on α-catenin. (a, b) Skin from wild-type and α-catenin knockout E18.5 mouse embryos were labelled with monospecific antibodies against formin-1 (IV) and either β4 integrin, to mark the epidermal (epi)–dermal (de) boundary (a, b), or E-cadherin, the transmembrane component of adherens junctions (a′, b′). Note cell–cell border staining in wild-type epidermis, but a more diffuse staining pattern in knockout epidermis. Some anti-formin-1 (IV) labelling was also detected in dermal cells. (c–n) Wild-type and _α-catenin_-null (KO) keratinocytes were cultured at high density overnight from the skins of newborn mice. At time zero, calcium was added to induce cell–cell adhesion, and at the times indicated thereafter. Cells were fixed before immunofluorescence staining with phalloidin, or antibodies against formin-1 (IV) or vinculin (Vinc), as indicated. After 8 h, adherens junctions are organized into distinctive rows of puncta (adhesion zippers), each with an associated radial cable of actin. After 24 h, an epithelial sheet has formed, with uniform adherens junction labelling at cell–cell borders. Note that formin-1 antibodies localize to puncta, radial actin cables and mature cell–cell borders. Note that formin-1 organization is perturbed in the absence of α-catenin, as are radial actin cables and adherens junctions. Notably, however, the overall actin cytoskeleton is largely intact in the knockout keratinocytes. Scale bars represent 10 μm.
Figure 3
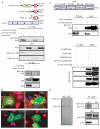
Formin-1 and α-catenin interact specifically. (a) The top panel shows a schematic representation of Flag-tagged ΔC-formin-1 (IV) and ΔC-formin-1 (V), formin-1-cc (N-terminally GFP-tagged α-catenin-binding domain) and α-cat–Myc (full-length α-catenin with a C-terminal Myc tag). The middle panel shows CMV-driven expression of Flag-tagged ΔC-formin-1 (IV) and ΔC-formin-1 (V). The bottom panel shows CMV-driven expression of GFP-tagged formin-1-cc (142 amino acids). 48 h after transfection, protein extracts were subjected to immunoprecipitation (IP) with the indicated antibodies. Total protein lysates (input) and immunoprecipitated products were analysed by western blotting. Co-expressed transgenes were always expressed at reduced levels relative to singly expressed transgenes. (b) Wild-type and _α-catenin_-null (KO) keratinocytes expressing CMV-formin-1-cc were either treated with high levels of calcium (right panels) or left untreated (left panels). Cells were then analysed by indirect immunofluorescence microscopy. Scale bar denotes 10 μm. (c) The top panel shows a schematic representation of the structural domains of α-catenin-. β-cat-BD, β-catenin-binding domain; DimD, dimerization domain; Vin/α-actBD, vinculin- and α-actinin-binding domains; AdhModD, adhesion modulation domain; Z01/Vin/α-act-BD, Z01-, vinculin- and α-actinin-binding domains. Each domain was Myc-tagged and expressed transiently with ΔC-formin-1 (IV). Immunoprecipitation and western blotting were performed as in a. Only the vinculin/α-actinin domain of α-catenin associated specifically with ΔC-formin-1 (IV). (d) Primary keratinocytes were lysed and subjected to anti-α-catenin immunoprecipitations. Samples were analysed by SDS–PAGE and western blotting with anti-formin-1 (IV) or anti-α catenin antibodies. Total lysates from wild-type and knockout keratinocytes were also subjected to anti-formin-1 (IV) western blotting.The formin-1 (IV) antibody is highly specific and detects a single band.
Figure 4
Formin-1 (FH1-FH2) nucleates actin filaments in vitro. (a) A schematic representation of GST–formin-1 fusion proteins used for these studies. (b) Coomassie-blue-stained SDS–PAGE gels and anti-GST western blots of bacterially expressed and purified proteins. M, molecular markers. (c) Nucleation of actin filaments by domains of formin-1, as determined by the pyrene–actin assembly assay. Assembly reactions contained 4 μM actin (10% pyrene actin) and formin-1 (FH1-FH2) between 10 and 200 nM. (d) Assembly reactions were performed as in c, except that the formin-1 (FH1-FH2) concentration was maintained at 100 nM and the actin concentration varied between 0 and 4 μM. (e) Time course of depolymerization of 5 μM actin filaments (10% pyrene-labelled) after dilution to 0.1 μM in the presence of 200 nM formin-1 (FH1-FH2), cytochalasin D (CytD), or actin alone, as indicated. (f) The effects of profilin, cytochalasin D, ld/ld truncation (Ld mut), removal of the FH2 domain (ΔFH2), ΔC-formin-1 and α-catenin on formin-1-induced actin polymerization. The formin-1 fragments in a were added to 4 μM pyrene–actin and filament assembly was monitored. For formin-1 (FH1-FH2) reactions were also conducted in the presence or absence of 5 μM profilin or 100 nM cytochalasin D. (g–j) Epifluorescence imaging of aliquots of select assembly reactions from c–f as indicated. (k) Electron micrograph of assembly reaction containing 100 nM formin-1 (FH1-FH2), 4 μM actin (10% pyrene–actin) and 5 μM profilin. (l) The same reaction as in k performed in the presence of cytochalasin D.
Figure 5
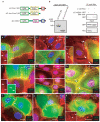
The formin-1 α-cat-BD perturbs intercellular junctions. (a) A schematic representation of the GFP fusion proteins used in this study. (b) The left panels shows an anti-GFP western blot to verify the size and stable expression of the proteins. The right panel shows immunoprecipitations, demonstrating that ΔC-mDia1, the mDia1 equivalent of ΔC-formin-1 (IV), does not bind to α-catenin. (c–k) Transient expression of ΔC-formin-1 (IV) (c–e), ΔC-Δcc-formin-1 (IV) (f–h) or ΔC-mDia1 (i–k) in primary, wild-type, keratinocytes. High-calcium medium was added 24 h after transfection. After a further 2.5 or 24 h, cells were analysed by indirect immunofluorescence microscopy with the indicated antibodies or with rhodamine–phalloidin. Boxed areas are shown at higher magnification in insets. Opposing arrows in c and asterisks in d denote disruption of cell–cell junctions between two cells transfected with ΔC-formin-1. Arrows and arrowheads in e depict puncta-associated radial actin cables present only in untransfected cells and not in transfected cells. Opposing arrows in f–k denote cell–cell junctions between two cells transfected with ΔC-Δcc-formin-1 or ΔC-mDia1. Arrowheads in k denote radial actin cables still present in a ΔC-mDia1-transfected cell. Scale bars in c–k represent 10 μm.
Figure 6
Rescuing intercellular junctions in _α-catenin_-null keratinocytes. (a) A schematic representation of the GFP fusion proteins used in this study. (b) Western blot and co-immunoprecipitation analyses, as described in Fig. 1, showing that the proteins are stably expressed and of the predicted sizes, and that they associate with β-catenin. (c–l) Rescue experiments. _α-catenin_-null (KO) keratinocytes were transfected as indicated in low-calcium medium. After 24 h, cultures were shifted to high-calcium medium for the indicated times before analysis by indirect immunofluorescence microscopy. The antibodies used are indicated. Arrows in c and d denote cell–cell borders. The cell borders in c, e, f and i lack proper adherens junctions. Scale bars in c–l represent 10 μm.
Comment in
- Formin' adherens junctions.
Zigmond S. Zigmond S. Nat Cell Biol. 2004 Jan;6(1):12-4. doi: 10.1038/ncb0104-12. Nat Cell Biol. 2004. PMID: 14704674 No abstract available.
Similar articles
- Formin' adherens junctions.
Zigmond S. Zigmond S. Nat Cell Biol. 2004 Jan;6(1):12-4. doi: 10.1038/ncb0104-12. Nat Cell Biol. 2004. PMID: 14704674 No abstract available. - The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin.
Kovar DR, Kuhn JR, Tichy AL, Pollard TD. Kovar DR, et al. J Cell Biol. 2003 Jun 9;161(5):875-87. doi: 10.1083/jcb.200211078. J Cell Biol. 2003. PMID: 12796476 Free PMC article. - Formin-mediated actin polymerization at cell-cell junctions stabilizes E-cadherin and maintains monolayer integrity during wound repair.
Rao MV, Zaidel-Bar R. Rao MV, et al. Mol Biol Cell. 2016 Sep 15;27(18):2844-56. doi: 10.1091/mbc.E16-06-0429. Epub 2016 Jul 20. Mol Biol Cell. 2016. PMID: 27440924 Free PMC article. - Review of the mechanism of processive actin filament elongation by formins.
Paul AS, Pollard TD. Paul AS, et al. Cell Motil Cytoskeleton. 2009 Aug;66(8):606-17. doi: 10.1002/cm.20379. Cell Motil Cytoskeleton. 2009. PMID: 19459187 Free PMC article. Review. - Fifteen formins for an actin filament: a molecular view on the regulation of human formins.
Schönichen A, Geyer M. Schönichen A, et al. Biochim Biophys Acta. 2010 Feb;1803(2):152-63. doi: 10.1016/j.bbamcr.2010.01.014. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20102729 Review.
Cited by
- Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells.
Li L, Bennett SA, Wang L. Li L, et al. Cell Adh Migr. 2012 Jan-Feb;6(1):59-70. doi: 10.4161/cam.19583. Cell Adh Migr. 2012. PMID: 22647941 Free PMC article. Review. - A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens.
Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. Verma S, et al. Mol Biol Cell. 2012 Dec;23(23):4601-10. doi: 10.1091/mbc.E12-08-0574. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051739 Free PMC article. - Adherens junctions in myelinating Schwann cells stabilize Schmidt-Lanterman incisures via recruitment of p120 catenin to E-cadherin.
Tricaud N, Perrin-Tricaud C, Brusés JL, Rutishauser U. Tricaud N, et al. J Neurosci. 2005 Mar 30;25(13):3259-69. doi: 10.1523/JNEUROSCI.5168-04.2005. J Neurosci. 2005. PMID: 15800180 Free PMC article. - Intercellular junction assembly, dynamics, and homeostasis.
Green KJ, Getsios S, Troyanovsky S, Godsel LM. Green KJ, et al. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a000125. doi: 10.1101/cshperspect.a000125. Cold Spring Harb Perspect Biol. 2010. PMID: 20182611 Free PMC article. Review. - Modulation of the tumor suppressor protein alpha-catenin by ischemic microenvironment.
Plumb CL, Adamcic U, Shahrzad S, Minhas K, Adham SA, Coomber BL. Plumb CL, et al. Am J Pathol. 2009 Oct;175(4):1662-74. doi: 10.2353/ajpath.2009.090007. Epub 2009 Sep 10. Am J Pathol. 2009. PMID: 19745064 Free PMC article.
References
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–576. - PubMed
- Welch MD, Rosenblatt J, Skoble J, Portnoy DA, Mitchison TJ. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. - PubMed
- Welch MD, Mullins RD. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 2002;18:247–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous