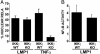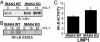Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6 - PubMed (original) (raw)
. 2003 Dec 23;100(26):15595-600.
doi: 10.1073/pnas.2136756100. Epub 2003 Dec 12.
Efthimios Prinarakis, Teruhito Yasui, Theodore Tsichritzis, Ellen Cahir-McFarland, Jun-Ichiro Inoue, Hiroyasu Nakano, Tak Wah Mak, Wen-Chen Yeh, Xiaoxia Li, Shizuo Akira, Nobutaka Suzuki, Shinobu Suzuki, George Mosialos, Elliott Kieff
Affiliations
- PMID: 14673102
- PMCID: PMC307613
- DOI: 10.1073/pnas.2136756100
Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6
Micah Luftig et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Epstein-Barr virus latent membrane protein 1 (LMP1) activation of NF-kappaB is critical for Epstein-Barr virus-infected B lymphocyte survival. LMP1 activates the IkappaB kinase complex and NF-kappaB through two cytoplasmic signaling domains that engage tumor necrosis factor receptor-associated factor (TRAF)1/2/3/5 or TRADD and RIP. We now use cells lacking expression of TRAF2, TRAF5, TRAF6, IKKalpha, IKKbeta, IKKgamma, TAB2, IL-1 receptor-associated kinase (IRAK)1, or IRAK4 to assess their roles in LMP1-mediated NF-kappaB activation. LMP1-induced RelA nuclear translocation was similar in IKKalpha knockout (KO) and WT murine embryo fibroblasts (MEFs) but substantially deficient in IKKbeta KO MEFs. NF-kappaB-dependent promoter responses were also substantially deficient in IKKbeta KO MEFs but were hyperactive in IKKalpha KO MEFs. More surprisingly, NF-kappaB responses were near normal in TRAF2 and TRAF5 double-KO MEFs, IKKgamma KO MEFs, TAB2 KO MEFs, and IRAK4 KO MEFs but were highly deficient in TRAF6 KO MEFs and IRAK1 KO HEK293 cells. Consistent with the importance of TRAF6, LMP1-induced NF-kappaB activation in HEK293 cells was inhibited by expression of dominant-negative TAB2 and Ubc13 alleles. These data extend a role for IKKalpha in IKKbeta regulation, identify an unusual IKKbeta-dependent and IKKgamma-independent NF-kappaB activation, and indicate that IRAK1 and TRAF6 are essential for LMP1-induced NF-kappaB activation.
Figures
Fig. 1.
The role of IKKα and IKKβ in LMP1-mediated NF-κB activation in MEFs. (A) WT, IKKα KO, and IKKβ KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 is shown. Average values ± SD are shown from three experiments, in which LMP1 expression was similar in all transfected MEFs (not shown). (B) WT, IKKα KO, and IKKβ KO MEFs were transfected with 3XκBL and pGK-β-gal alone or with LMP1. The mean folds of NF-κB activation by LMP1 ± SE relative to β-gal activity are shown from one representative of four independent experiments performed in duplicate. (C) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of WT IKKβ expression vector in IKKβ KO MEFs are shown from a representative transfection. (D) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of catalytically inactive IKKβ (M-IKKβ) in IKKβ KO MEFs are shown from a representative transfection. (E) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of catalytically inactive IKKα (M-IKKα) in IKKα KO MEFs. (F) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of WT IKKα expression vector in IKKα KO MEFs.
Fig. 2.
IKKγ is not essential for LMP1-mediated NF-κB activation. (A) IKKγ WT and KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid or TNF stimulation. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 or TNF is shown. Average values ± SD are shown from three experiments. LMP1 expression was similar in WT and KO MEFs (data not shown). (B) IKKγ WT and KO MEFs were transfected with reporter plasmids alone or with LMP1. Mean folds of NF-κB activation ± SD by LMP1 are shown from four experiments.
Fig. 3.
TRAF2 and TRAF5 are not essential for LMP1-mediated NF-κB activation in MEFs. (A) WT and T2/5KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid or TNF stimulation. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 or TNF is shown. Average values ± SD are shown from three experiments, except from LMP1-mediated translocation of GFP-RelA in T2/5KO MEFs, for which five experiments were analyzed. LMP1 expression in WT and KO MEFs was similar (data not shown). (B) WT and T2/5KO MEFs were transfected with reporter plasmids alone or with an LMP1 plasmid. The mean ± SD of folds of NF-κB activation by LMP1 from four experiments is shown.
Fig. 4.
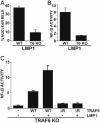
TRAF6 is essential for LMP1-mediated NF-κB activation in MEFs. (A)WT and TRAF6 KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 is shown. Average values ± SD are shown from three experiments. LMP1 expression was similar in WT and KO MEFs (data not shown). (B) WT and TRAF6 KO MEFs were transfected with reporter plasmids alone or with an LMP1 plasmid. Mean ± SE of folds of NF-κB activation by LMP1 are shown from one representative of four experiments performed in duplicate. (C) TRAF6 KO MEFs were transfected with reporter plasmids, LMP1, and WT, or ΔRF mTRAF6 expression plasmids as indicated. Results from one representative of four similar experiments are shown as mean folds of NF-κB activation by LMP1 and/or TRAF6 ± SE.
Fig. 5.
LMP1-mediated NF-κB activation is inhibited by TAB2-C and Ubc13-C87A but is not dependent on TAB2. (A) 293T cells were transfected with reporter plasmids alone, an LMP1 expression plasmid, or LMP1 and increasing amounts of TAB2-C expression plasmid (1 or 2 μg). Results from one representative transfection of two experiments show the mean folds of NF-κB activation by LMP1. (B)WT and TAB2 KO MEFs were treated with 100 ng/ml mIL-1 for 15 min or left untreated. Cell lysates were blotted for endogenous IκBα (Santa Cruz Biotechnology, C-21). (C) WT and TAB2 KO MEFs were transfected with reporter plasmids alone or with an LMP1 expression plasmid. Results from one representative transfection performed in triplicate show the mean ± SE of folds of NF-κB activation by LMP1. (D) An experiment similar to A was performed except that 1μg of Ubc13-C87A (M-UBC13) was cotransfected with LMP1. Results from one representative transfection of several experiments performed in duplicate show the mean NF-κB activation by LMP1. LMP1 was expressed at similar levels in the presence or absence of Ubc13-C87A (data not shown).
Fig. 6.
IRAK4 is not essential for LMP1-mediated NF-κB activation in MEFs. (A) IRAK4 WT and IRAK4 KO MEFs were treated with 100 ng/ml mIL-1 for 15 min or left untreated. Cell lysates were blotted for endogenous IκBα.(B) Cells were left untreated or treated with mIL-1 as in A and harvested at 15 and 30 min for electrophoretic mobility-shift assay. (C) IRAK4 WT or KO MEFs were transfected with reporter plasmids alone or a LMP1 expression plasmid. Results from one representative transfection of two performed in duplicate show the mean ± SE of folds of NF-κB activation by LMP1. LMP1 was expressed at similar levels in WT and KO MEFs (data not shown).
Fig. 7.
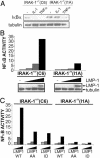
IRAK1 is critical for LMP1-mediated NF-κB activation in 293 cells. (A) C6 or I1A cells were incubated with 100 ng/ml human TNF or human IL-1 and harvested 30 min later for immunoblotting for IκBα and tubulin (Sigma, C-2-1-5). (B) C6 and I1A cells were transfected with reporter plasmids alone or with 0, 0.1, 1, or 5 μg of an LMP1 expression plasmid and harvested 24 h later for immunoblotting and luciferase and β-gal assays. Results show NF-κB activation by LMP1 and LMP1 and tubulin expression levels. (C) Reporter assays were performed as in B except that, in addition to WT LMP1, AA and ID mutants were transfected in identical increasing amounts into C6 and I1A cells. Results show relative NF-κB activation by LMP1 alleles.
References
- Cahir-McFarland, E. D., Izumi, K. M. & Mosialos, G. (1999) Oncogene 18, 6959–6964. - PubMed
- Rickinson, A. & Kieff, E. (2001) in Fields Virology, eds. Howley, P. M. & Knipe, D. (Lippincott, Philadelphia) pp. 2575–2627.
- Wang, D., Liebowitz, D. & Kieff, E. (1985) Cell 43, 831–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA87661/CA/NCI NIH HHS/United States
- R01 CA047006/CA/NCI NIH HHS/United States
- CA85180/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- R35 CA047006/CA/NCI NIH HHS/United States
- CA47006/CA/NCI NIH HHS/United States
- R01 CA085180/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous