The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation - PubMed (original) (raw)
. 2003 Dec;112(12):1887-94.
doi: 10.1172/JCI19757.
Mohammed A Khan, Mark Ambrose, Olga Nikolayeva, Meng Xu-Welliver, Maria Kartalou, S Perwez Hussain, Richard B Roth, Xiaoling Zhou, Leah E Mechanic, Irit Zurer, Varda Rotter, Leona D Samson, Curtis C Harris
Affiliations
- PMID: 14679184
- PMCID: PMC296999
- DOI: 10.1172/JCI19757
The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation
Lorne J Hofseth et al. J Clin Invest. 2003 Dec.
Erratum in
- J Clin Invest. 2004 Feb;113(3):490
Abstract
Chronic infection and associated inflammation are key contributors to human carcinogenesis. Ulcerative colitis (UC) is an oxyradical overload disease and is characterized by free radical stress and colon cancer proneness. Here we examined tissues from noncancerous colons of ulcerative colitis patients to determine (a) the activity of two base excision-repair enzymes, AAG, the major 3-methyladenine DNA glycosylase, and APE1, the major apurinic site endonuclease; and (b) the prevalence of microsatellite instability (MSI). AAG and APE1 were significantly increased in UC colon epithelium undergoing elevated inflammation and MSI was positively correlated with their imbalanced enzymatic activities. These latter results were supported by mechanistic studies using yeast and human cell models in which overexpression of AAG and/or APE1 was associated with frameshift mutations and MSI. Our results are consistent with the hypothesis that the adaptive and imbalanced increase in AAG and APE1 is a novel mechanism contributing to MSI in patients with UC and may extend to chronic inflammatory or other diseases with MSI of unknown etiology.
Figures
Figure 1
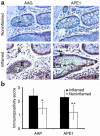
Cellular localization and quantification of AAG and APE1 in the UC colon. (a) AAG and APE1 were present in inflammatory cells (I) and in the mucosa (epithelium [E] and stroma [S]) of a patient with UC. Magnification, ×400. (b) AAG (n = 8) and APE1 (n = 8) levels were quantified in areas of UC colon pathologically defined as inflamed (black bars) and noninflamed (white bars). *, significantly lower AAG levels in the noninflamed areas of the UC colon (P = 0.04). **, significantly lower APE1 levels in the noninflamed areas of the UC colon (P = 0.003). Scoring criteria are described in Methods.
Figure 2
AAG and APE1 activities adaptively increase in response to increasing amounts of inflammation. (a) Examples of how levels of CD68 parallel changes in AAG activity. Tissues X and Y were categorized into inflamed (I) and noninflamed (NI) areas as described in Methods, then assessed for AAG activity (arrowhead). (b) Examples of how levels of CD68 parallel changes in APE1 activity. Tissues X and Y were categorized into inflamed and noninflamed areas as described in Methods, then assessed for APE1 activity (arrowhead). (c) Comparison of activity of AAG in areas of the UC colon categorized by CD68 levels as noninflamed or inflamed (n = 30). *, significant difference from CD68-low tissues (paired t test, P < 0.01). (d) Comparison of activity of APE1 in areas of the UC colon categorized by CD68 levels as noninflamed or inflamed. *, significant difference from CD68-low tissues (paired t-test, P < 0.01). (e) Pearson correlation coefficient quantifying the ratio of samples X/Y with regards to CD68 densitometry and AAG activity. (f) Pearson correlation coefficient quantifying the ratio of samples X/Y with regards to CD68 densitometry and APE1 activity.
Figure 3
(a and b) Correlation between MSI and AAG (a) or APE1 (b) activity. Bar graphs represent means ± SEM. There was a significant trend for MSI and AAG activity (robust regression analysis, P = 0.0012). Although this trend was not observed between MSI and APE1, there was a significant increase in APE1 activity in the MSI-High group (n = 5; one-way ANOVA with Scheffe multiple comparison test, P = 0.0004). *, AAG activity is significantly higher in the MSI-Low group (n = 10) than in the microsatellite stable group (n = 15). **, AAG activity is significantly higher in the MSI-High group (n = 5) than in the MSI-Low group (n = 10). ***, APE1 activity is significantly higher in the MSI-High group (n = 5) than in the MSI-Low (n = 10) and microsatellite stable (n = 15) groups. (c–e) Number of samples belonging to a specific AAG and APE1 activity category. AAG and APE1 activities were ranked in order, then placed into tertiles as samples with activity belonging to the Lower 1/3, Middle 1/3, or Top 1/3. (c) Of the 60 samples, 43 did not have a band shift and were characterized as microsatellite stable samples. (d) Of the 60 samples, 11 had a band shift in one of the markers examined (including TGFβRII and BLM) and were characterized as MSI-Low samples. (e) Of the 60 samples, six had a band shift in two or more of the markers examined (including TGFβRII and BLM) and were characterized as MSI-High samples. Shaded boxes represent activities where there is an imbalance of AAG and APE1 activities. The simple κ statistic indicates a trend for imbalance between AAG and APE1 as MSI levels increase. The simple κ statistic of 1.0 indicates no imbalance. A simple κ statistic moving toward zero indicates greater imbalance between the two enzymes.
Figure 4
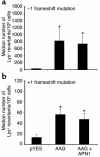
Induction of –1 (a) and +1 (b) frameshift mutations in yeast by human AAG. Plasmids pYES2.0 and pYES-AAG were transformed into the E133 or E134 yeast strains, then assessed for frameshift mutations by reversion from Lys– to Lys+. APN1 was also coexpressed to determine its effect on AAG-induced mutations (right bars). Bar graphs represent the mean ± standard deviations. *, significant difference from pYES control (P < 0.01).
Comment in
- Tumbling down a different pathway to genetic instability.
Guo HH, Loeb LA. Guo HH, et al. J Clin Invest. 2003 Dec;112(12):1793-5. doi: 10.1172/JCI20502. J Clin Invest. 2003. PMID: 14679175 Free PMC article. Review.
References
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 1990;323:1228–1233. - PubMed
- O’Sullivan JN, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 2002;32:280–284. - PubMed
- Brentnall TA, et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237–1240. - PubMed
- Chang CL, et al. Oxidative stress inactivates the human DNA mismatch repair system. Am. J. Physiol. Cell Physiol. 2002;283:C148–C154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA055042/CA/NCI NIH HHS/United States
- ES02109/ES/NIEHS NIH HHS/United States
- CA55042/CA/NCI NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
- CA75576/CA/NCI NIH HHS/United States
- R01 CA075576/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous