Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia - PubMed (original) (raw)
Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia
Tomohisa Suzuki et al. J Neurosci. 2004.
Abstract
After a brain insult, ATP is released from injured cells and activates microglia. The microglia that are activated in this way then release a range of bioactive substances, one of which is tumor necrosis factor (TNF). The release of TNF appears to be dependent on the P2X7 receptor. The inhibitors 1,4-diamino-2,3-dicyano-1,4-bis[2-amino-phenylthio]butadiene (U0126), anthra[1,9-cd]pyrazol-6(2H)-one (SP600125), and 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)IH-imidazole (SB203580), which target MEK (mitogen-activated protein kinase kinase), JNK (c-Jun N-terminal kinase), and p38, respectively, all potently suppress the production of TNF in ATP-stimulated microglia, whereas the production of TNF mRNA is strongly inhibited by U0126 and SP600125. SB203580 did not affect the increased levels of TNF mRNA but did prevent TNF mRNA from accumulating in the cytoplasm. The ATP-provoked activation of JNK and p38 [but not extracellular signal-regulated kinase (ERK)] could be inhibited by brilliant blue G, a P2X7 receptor blocker, and by genistein and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine, which are general and src-family-specific tyrosine kinase inhibitors, respectively. Most important, we found that treatment of the microglia in neuron-microglia cocultures with the P2X7 agonist 2'-3'-O-(benzoyl-benzoyl) ATP led to significant reductions in glutamate-induced neuronal cell death, and that either TNF-alpha converting enzyme inhibitor or anti-TNF readily suppressed the protective effect implied by this result. Together, these findings indicate that both ERK and JNK are involved in the regulation of TNF mRNA expression, that p38 is involved in the nucleocytoplasmic transport of TNF mRNA, and that a PTK (protein tyrosine kinase), possibly a member of the src family, acts downstream of the P2X7 receptor to activate JNK and p38. Finally, our data suggest that P2X7 receptor-activated microglia protect neurons against glutamate toxicity primarily because they are able to release TNF.
Figures
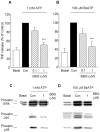
Figure 1.
Effects of U0126 (U), SP600125 (SP), and SB203580 (SB) on ATP-induced TNF release, intracellular TNF production, and mRNA expression in microglia. The cells were treated with 10 μ
m
U0126, 30 μ
m
SP600125, or 15 μ
m
SB203580 for 15 min and stimulated with 1 m
m
ATP for 3 hr (A), 2 hr (B), and 1 hr (C). The released TNF (A) and the intracellular TNF contents (B) were measured by ELISA. Values are expressed as mean ± SEM of percentage of release compared with ATP alone from three independent experiments. Values of 100% for the release and intracellular production of TNF in ATP-stimulated microglia were 178.8 ± 22.2 and 407.0 ± 45.6 pg/106 cells, respectively. C, The expression of TNF mRNA was quantified by real-time RT-PCR. Values are shown as the ratio of TNF mRNA versus GAPDH mRNA. Data are expressed as mean ± SEM of ratio of expression compared with ATP or BzATP alone from three independent experiments. **p < 0.01; ***p < 0.001, significantly different from the control (Con) (t test).
Figure 2.
Distribution of TNF mRNA induced by ATP in the nucleus and cytoplasm of microglia treated with SB203580 (SB). A, The cells were treated with 15 μ
m
SB203580 for 15 min and stimulated with 1 m
m
ATP for 1 hr. Nuclear and cytoplasmic fractions of the cells were separated by NE-PER kit, and RNA was extracted from each fraction. Values are shown as the ratio of TNF mRNA levels versus GAPDH mRNA levels. Data are expressed as mean ± SEM of ratio of expression compared with ATP alone from three independent experiments. *p < 0.05 significantly different from the control (Con) (t test). B, Nuclear and cytoplasmic extracts were separated by SDS-PAGE and probed with antibodies to Oct-1 [nuclear protein (n)] and Hsp-90 [cytoplasmic protein (c)] to determine the efficiency of nucleocytoplasmic separation.
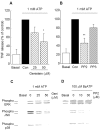
Figure 3.
Effects of BBG on the release of TNF and the activation of ERK, JNK, and p38 MAP kinase in ATP- or BzATP-stimulated microglia. The cells were treated with BBG (0.1 or 1 μ
m
) for 5 min and stimulated with 1 m
m
ATP (A, C) or 100 μ
m
BzATP (B, D) for 3 hr (A, B) or 10 min (C, D). A, B, The release of TNF was measured by ELISA. Values are expressed as mean ± SEM of percentage of release compared with ATP or BzATP alone from six independent experiments. Values for 100% of release of TNF were 183.8 ± 46.3 and 350.7 ± 152.2 pg/106 cells in ATP- or BzATP-stimulated microglia, respectively. *p < 0.05; ***p < 0.001, significantly different from the control (Con) (t test). C, D, The phosphorylated (active) and total ERK, JNK, and p38 were detected by Western blotting using antibodies that recognize phosphorylated and both phosphorylated and nonphosphorylated enzymes, respectively. The levels of each total MAPK were confirmed to be identical for each lane. Similar results were obtained in at least three independent experiments.
Figure 4.
Effects of genistein and PP2 on the release of TNF and the activation of ERK, JNK, and p38 MAP kinase induced by ATP. Microglia were treated with genistein, a nonselective PTK inhibitor (A, C), with PP2, an _src_-family-selective PTK inhibitor, or with PP3, an inactive analog of PP2 (B, D); stimulated with 1 m
m
ATP or 100 μ
m
BzATP; and then measured for the release of TNF after 3 hr (A, B) and MAP kinase activation after 10 min (C, D). Values are expressed as mean ± SEM of percentage of release compared with ATP alone from three independent experiments. Values of 100% for the release of TNF were 136.0 ± 37.0 (A) and 133.6 ± 65.1 (B) pg/106 cells. *p < 0.05; **p < 0.01, significantly different from the control (Con) (t test). C, D, The phosphorylated (Phospho) (active) and total ERK, JNK, and p38 were detected by Western blotting using antibodies that recognize either phosphorylated or both phosphorylated and unphosphorylated enzymes, respectively. The (total) levels of each MAP kinase were confirmed to be identical for each lane. Similar results were obtained in at least three independent experiments. Geni, Genistein.
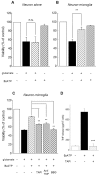
Figure 5.
Effects of BzATP on the viability of primary cortical neurons in “neuron alone” cultures or neuron-microglia cocultures. Primary cultures of rat cortical neurons alone (A) or cocultures of neurons with microglia (B) were treated with 100 μ
m
BzATP for 24 hr and then stimulated with 100 μ
m
glutamate for 10 min. After 24 hr of incubation, neuronal cell viability was determined by MTT assay. Values are expressed as mean ± SEM of percentage of viability of control cells from three independent experiments. **p < 0.01, significantly different from glutamate alone (t test). n.s., Not significant. C, Effects of TAPI, TACE inhibitor, anti-TNF, and BBG on BzATP-induced neuroprotection. Cortical neurons cocultured with microglia were treated with 50 μ
m
TAPI, 10 μg/ml anti-TNF, and 1 μ
m
BBG for 5 min before BzATP application. After 24 hr of coculture, the neurons were stimulated with 100 μ
m
glutamate for 10 min. After 24 hr of additional incubation, neuronal cell viability was determined by MTT assay. Values are expressed as mean ± SEM of percentage of viability of control cells from three independent experiments. **p < 0.01, significantly different from BzATP-glutamate application (t test). D, Effects of TAPI on BzATP-induced TNF release from rat microglia. ***p < 0.001.
Similar articles
- Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia.
Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Hide I, et al. J Neurochem. 2000 Sep;75(3):965-72. doi: 10.1046/j.1471-4159.2000.0750965.x. J Neurochem. 2000. PMID: 10936177 - P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways.
Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. Shiratori M, et al. J Neurochem. 2010 Aug;114(3):810-9. doi: 10.1111/j.1471-4159.2010.06809.x. Epub 2010 May 13. J Neurochem. 2010. PMID: 20477948 - P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase.
Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. Panenka W, et al. J Neurosci. 2001 Sep 15;21(18):7135-42. doi: 10.1523/JNEUROSCI.21-18-07135.2001. J Neurosci. 2001. PMID: 11549724 Free PMC article. - [Mechanism of production and release of tumor necrosis factor implicated in inflammatory diseases].
Hide I. Hide I. Nihon Yakurigaku Zasshi. 2003 Mar;121(3):163-73. doi: 10.1254/fpj.121.163. Nihon Yakurigaku Zasshi. 2003. PMID: 12673950 Review. Japanese. - P2X7 receptors in oligodendrocytes: a novel target for neuroprotection.
Matute C. Matute C. Mol Neurobiol. 2008 Oct;38(2):123-8. doi: 10.1007/s12035-008-8028-x. Epub 2008 Aug 14. Mol Neurobiol. 2008. PMID: 18704769 Review.
Cited by
- Neurotransmitter signaling in the pathophysiology of microglia.
Domercq M, Vázquez-Villoldo N, Matute C. Domercq M, et al. Front Cell Neurosci. 2013 Apr 19;7:49. doi: 10.3389/fncel.2013.00049. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23626522 Free PMC article. - Lack of functional P2X7 receptor aggravates brain edema development after middle cerebral artery occlusion.
Kaiser M, Penk A, Franke H, Krügel U, Nörenberg W, Huster D, Schaefer M. Kaiser M, et al. Purinergic Signal. 2016 Sep;12(3):453-63. doi: 10.1007/s11302-016-9511-x. Epub 2016 Apr 5. Purinergic Signal. 2016. PMID: 27048203 Free PMC article. - P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease.
Shokoples BG, Paradis P, Schiffrin EL. Shokoples BG, et al. Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):186-199. doi: 10.1161/ATVBAHA.120.315116. Epub 2020 Oct 1. Arterioscler Thromb Vasc Biol. 2021. PMID: 32998520 Free PMC article. Review. - Transient changes in the localization and activity of ecto-nucleotidases in rat hippocampus following lipopolysaccharide treatment.
Kittel A, Sperlágh B, Pelletier J, Sévigny J, Kirley TL. Kittel A, et al. Int J Dev Neurosci. 2007 Aug;25(5):275-82. doi: 10.1016/j.ijdevneu.2007.05.007. Epub 2007 May 17. Int J Dev Neurosci. 2007. PMID: 17576046 Free PMC article. - Early P2X7R-dependent activation of microglia during the asymptomatic phase of autoimmune encephalomyelitis.
Grygorowicz T, Strużyńska L. Grygorowicz T, et al. Inflammopharmacology. 2019 Feb;27(1):129-137. doi: 10.1007/s10787-018-0528-3. Epub 2018 Sep 12. Inflammopharmacology. 2019. PMID: 30209761 Free PMC article.
References
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP (2001) TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4: 1116-1122. - PubMed
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ (1997) Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 28: 1233-1244. - PubMed
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A (2001) CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat Neurosci 4: 702-710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous