Receptor clustering is involved in Reelin signaling - PubMed (original) (raw)
. 2004 Feb;24(3):1378-86.
doi: 10.1128/MCB.24.3.1378-1386.2004.
Daniela Fasching, Christoph Hauser, Harald Mayer, Hans H Bock, Thomas Hiesberger, Joachim Herz, Edwin J Weeber, J David Sweatt, Albéna Pramatarova, Brian Howell, Wolfgang J Schneider, Johannes Nimpf
Affiliations
- PMID: 14729980
- PMCID: PMC321426
- DOI: 10.1128/MCB.24.3.1378-1386.2004
Receptor clustering is involved in Reelin signaling
Vera Strasser et al. Mol Cell Biol. 2004 Feb.
Abstract
The Reelin signaling cascade plays a crucial role in the correct positioning of neurons during embryonic brain development. Reelin binding to apolipoprotein E receptor 2 (ApoER2) and very-low-density-lipoprotein receptor (VLDLR) leads to phosphorylation of disabled 1 (Dab1), an adaptor protein which associates with the intracellular domains of both receptors. Coreceptors for Reelin have been postulated to be necessary for Dab1 phosphorylation. We show that bivalent agents specifically binding to ApoER2 or VLDLR are sufficient to mimic the Reelin signal. These agents induce Dab1 phosphorylation, activate members of the Src family of nonreceptor tyrosine kinases, modulate protein kinase B/Akt phosphorylation, and increase long-term potentiation in hippocampal slices. Induced dimerization of Dab1 in HEK293 cells leads to its phosphorylation even in the absence of Reelin receptors. The mechanism for and the sites of these phosphorylations are identical to those effected by Reelin in primary neurons. These results suggest that binding of Reelin, which exists as a homodimer in vivo, to ApoER2 and VLDLR induces clustering of ApoER2 and VLDLR. As a consequence, Dab1 becomes dimerized or oligomerized on the cytosolic side of the plasma membrane, constituting the active substrate for the kinase; this process seems to be sufficient to transmit the signal and does not appear to require any coreceptor.
Figures
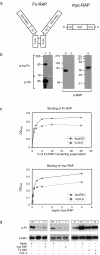
FIG. 1.
A dimeric form of RAP induces tyrosine phosphorylation of Dab1 in primary embryonic neurons. (a) Structure of the dimeric Fc-RAP and the monomeric myc-RAP. hu-Fc, human Fc. (b) 293T cells were transfected with the cDNA encoding Fc-RAP. Ten microliters of the respective cell supernatant (1, 3) as well as 10 μl of control supernatant (2, 4) was analyzed by Western blotting using the indicated antibodies. myc-RAP expressed in E. coli (5) was analyzed by Western blotting using an anti-RAP antibody. Molecular size markers are shown to the left of each panel. a-hu Fc, anti-human Fc; a-V5, anti-V5; a-RAP, anti-RAP. (c) Microtiter plates were coated with ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His. After incubationwith the indicated amounts of Fc-RAP (top) or myc-RAP (bottom), bound Fc-RAP was detected with protein A-HRP and myc-RAP was detected with 9E10 and an appropriate HRP-conjugated secondary antibody. OD450, optical density at 450 nm. (d) Primary mouse neurons were incubated with different conditioned or control media as indicated. myc-RAP was used at a concentration of 100 μg/ml. Cells were processed for immunoprecipitation with anti-Dab1 antibody, and Western blotting was subsequently performed using antiphosphotyrosine (a-PY) and anti-Dab1 (a-Dab1) antibodies. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by Reelin was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels. Prot. A, protein A; +, present; −, absent.
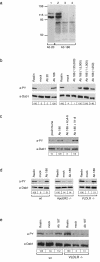
FIG. 2.
Polyclonal antibodies directed against the ligand binding domains of ApoER2 and VLDLR induce Dab1 phosphorylation in primary embryonic neurons. (a) 293T cells were transfected with constructs encoding ApoER2 (ApoER2Δ4-6) (lanes 1 and 2) or VLDLR (lane 3) or with the empty vector (lane 4). The corresponding cell extracts were separated using SDS-8% PAGE under reducing conditions, and Western blotting was performed using Ab 20 (lane 1) or Ab 186 (lanes 2 to 4). Molecular size markers are shown at the left. (b) Primary mouse neurons were incubated with Reelin-conditioned medium, control medium, or antiserum 20 or 186 (1:1,000) (left panel). Neurons were stimulated with different concentrations of Ab 186 (right panel). Cells were processed for immunoprecipitation with anti-Dab1,and Western blotting was subsequently performed using antiphosphotyrosine (a-PY) or anti-Dab1 (a-Dab1) antibody as indicated. (c) Primary neurons were incubated with the preimmune serum from the rabbit producing Ab 186 or with Ab 186 alone or in the presence of an excess of soluble ligand binding domains of ApoER2 (AΔ4-6) or VLDLR (V1-8). Immunoprecipitation and Western blot analysis were performed as described for panel b. (d) Primary neurons from wt, ApoER2−/−, and VLDLR−/− mice were stimulated with Reelin, control medium, or Ab 186 (1:1,000). Immunoprecipitation and Western blot analysis were performed as described for panel b. (e) Primary mouse neurons derived from wt or VLDLR−/− mice were incubated with Reelin-conditioned medium, control medium, or serum from the rabbit producing Ab 187 at a dilution of 1:800. Cells were processed for immunoprecipitation with anti-Dab1 antibody, and Western blotting was subsequently performed using anti-PY and anti-Dab1 antibodies as indicated. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by Reelin was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels.
FIG. 3.
Ab 186 induces activation of SFKs and PKB/Akt in primary embryonic neurons. Primary rat neurons were incubated with Reelin-conditioned medium, control medium, or Ab 186, and the phosphorylation of Dab1, SFKs, and PKB/Akt was analyzed by Western blotting of crude cell lysates using the indicated antibodies. The IgG heavy chain marked by the arrow in the phospho-Akt immunoblot originates from Ab 186 added in this experiment. Molecular size markers are shown at the left. a-PY, antiphosphotyrosine; p-Dab1, phospho-Dab1; a-Dab1, anti-Dab1; a-p-SFK, anti-phospho-SFK; a-Fyn, anti-Fyn; a-p-AKT, anti-phospho-Akt; a-AKT, anti-Akt.
FIG. 4.
Perfusion with Fc-RAP enhances hippocampal LTP induction. Hippocampal slices were perfused with Fc-RAP (10 μg/ml), Fc (10 μg/ml), or control medium. Baseline synaptic responses (a) and potentiation immediately following HFS (b) and up to 60 min after HFS (c) were recorded. The arrowhead represents LTP induced with two trains of 1-s-long, 100-Hz stimulation, separated by 20 s. The horizontal line indicates application of Fc-RAP, Fc, or control medium. Results are shown as means ± standard errors of the mean. fEPSP, field excitatory postsynaptic potential.
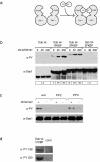
FIG. 5.
Dimerization and oligomerization of Dab1 lead to receptor-independent phosphorylation in 293 cells. (a) Cartoon demonstrating dimerization of the Dab1-FKBP fusion protein by addition of AP20187. (b) Wt Dab1, fusion proteins containing wt Dab1 and one (Dab wt-1FKBP) or two (Dab wt-2FKBP) copies of FKBP, and a fusion protein containing a mutated version of Dab1 with the relevant five tyrosine residues replaced by phenylalanines and containing two copies of FKBP (Dab 5F-2FKBP) were expressed in 293 cells and treated with the indicated amounts of the FKBP-dimerizing agent AP20187 for 20 min. Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. a-PY, antiphosphotyrosine; a-Dab1, anti-Dab1; n.d., not determined. (c) 293 cells expressing Dab wt-1FKBP were treated for 1 h with or without (w/o) 10 μM PP2 or PP3 prior to the addition of 50 nM AP20187 or medium. After 20 min, Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. +, present; −, absent. (d) 293 cells expressing Dab wt-1FKBP or control cells (contr.) were treated with 50 nM AP20187, and after 20 min Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by the highest concentration of AP20187 was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels.
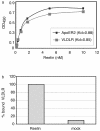
FIG. 6.
Reelin acts as a multivalent ligand for ApoER2 and VLDLR. (a) Microtiter plates were coated with ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His (10 μg/ml). After incubation with the indicated amounts of Reelin, bound protein was detected with anti-Reelin antibody (G10) and HRP-conjugated secondary antibody as described in Materials and Methods. OD450, optical density at 450 nm; Kd, dissociation constant. (b) Microtiter plates were coated with ApoER2Δ4-6-MBP/His and incubated with Reelin (200 nM) or concentrated mock medium. After the plates were washed, VLDLR1-8-MBP/His (10 μg/ml) was added and bound VLDLR was detected using a monoclonal anti-VLDLR antibody (6G6) and HRP-conjugated secondary antibody.
Similar articles
- Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1.
Benhayon D, Magdaleno S, Curran T. Benhayon D, et al. Brain Res Mol Brain Res. 2003 Apr 10;112(1-2):33-45. doi: 10.1016/s0169-328x(03)00032-9. Brain Res Mol Brain Res. 2003. PMID: 12670700 - Reelin activates SRC family tyrosine kinases in neurons.
Bock HH, Herz J. Bock HH, et al. Curr Biol. 2003 Jan 8;13(1):18-26. doi: 10.1016/s0960-9822(02)01403-3. Curr Biol. 2003. PMID: 12526740 - Dab1-mediated colocalization of multi-adaptor protein CIN85 with Reelin receptors, ApoER2 and VLDLR, in neurons.
Fuchigami T, Sato Y, Tomita Y, Takano T, Miyauchi SY, Tsuchiya Y, Saito T, Kubo K, Nakajima K, Fukuda M, Hattori M, Hisanaga S. Fuchigami T, et al. Genes Cells. 2013 May;18(5):410-24. doi: 10.1111/gtc.12045. Epub 2013 Mar 19. Genes Cells. 2013. PMID: 23506116 - The functions of Reelin in membrane trafficking and cytoskeletal dynamics: implications for neuronal migration, polarization and differentiation.
Santana J, Marzolo MP. Santana J, et al. Biochem J. 2017 Sep 7;474(18):3137-3165. doi: 10.1042/BCJ20160628. Biochem J. 2017. PMID: 28887403 Review. - Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation.
Jossin Y. Jossin Y. Biomolecules. 2020 Jun 26;10(6):964. doi: 10.3390/biom10060964. Biomolecules. 2020. PMID: 32604886 Free PMC article. Review.
Cited by
- The Dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide-exchange activity.
Baumeister MA, Rossman KL, Sondek J, Lemmon MA. Baumeister MA, et al. Biochem J. 2006 Dec 15;400(3):563-72. doi: 10.1042/BJ20061020. Biochem J. 2006. PMID: 17007612 Free PMC article. - Structure of a receptor-binding fragment of reelin and mutational analysis reveal a recognition mechanism similar to endocytic receptors.
Yasui N, Nogi T, Kitao T, Nakano Y, Hattori M, Takagi J. Yasui N, et al. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):9988-93. doi: 10.1073/pnas.0700438104. Epub 2007 Jun 4. Proc Natl Acad Sci U S A. 2007. PMID: 17548821 Free PMC article. - Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity.
Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J. Beffert U, et al. J Neurosci. 2006 Feb 15;26(7):2041-52. doi: 10.1523/JNEUROSCI.4566-05.2006. J Neurosci. 2006. PMID: 16481437 Free PMC article. - Similarities and differences between the Wnt and reelin pathways in the forming brain.
Reiner O, Sapir T. Reiner O, et al. Mol Neurobiol. 2005;31(1-3):117-34. doi: 10.1385/MN:31:1-3:117. Mol Neurobiol. 2005. PMID: 15953816 Review. - Regulation of the hippocampal translatome by Apoer2-ICD release.
Wasser CR, Werthmann GC, Hall EM, Kuhbandner K, Wong CH, Durakoglugil MS, Herz J. Wasser CR, et al. Mol Neurodegener. 2023 Sep 19;18(1):62. doi: 10.1186/s13024-023-00652-1. Mol Neurodegener. 2023. PMID: 37726747 Free PMC article.
References
- Andersen, O. M., D. Benhayon, T. Curran, and T. E. Willnow. 2003. Differential binding of ligands to the apolipoprotein E receptor 2. Biochemistry 42:9355-9364. - PubMed
- Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr. Biol. 13:9-17. - PubMed
- Ballif, B. A., L. Arnaud, and J. A. Cooper. 2003. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Mol. Brain Res. 117:152-159. - PubMed
- Beffert, U., G. Morfini, H. H. Bock, H. Reyna, S. T. Brady, and J. Herz. 2002. Reelin-mediated signaling locally regulates PKB/Akt and GSK-3β. J. Biol. Chem. 277:49958-49964. - PubMed
- Benhayon, D., S. Magdaleno, and T. Curran. 2003. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Mol. Brain Res. 112:33-45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous