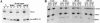Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs - PubMed (original) (raw)
Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs
Markus T Bohnsack et al. RNA. 2004 Feb.
Abstract
microRNAs (miRNAs) are widespread among eukaryotes, and studies in several systems have revealed that miRNAs can regulate expression of specific genes. Primary miRNA transcripts are initially processed to approximately 70-nucleotide (nt) stem-loop structures (pre-miRNAs), exported to the cytoplasm, further processed to yield approximately 22-nt dsRNAs, and finally incorporated into ribonucleoprotein particles, which are thought to be the active species. Here we study nuclear export of pre-miRNAs and show that the process is saturable and thus carrier-mediated. Export is sensitive to depletion of nuclear RanGTP and, according to this criterion, mediated by a RanGTP-dependent exportin. An unbiased affinity chromatography approach with immobilized pre-miRNAs identified exportin 5 as the pre-miRNA-specific export carrier. We have cloned exportin 5 from Xenopus and demonstrate that antibodies raised against the Xenopus receptor specifically block pre-miRNA export from nuclei of Xenopus oocytes. We further show that exportin 5 interacts with double-stranded RNA in a sequence-independent manner.
Figures
FIGURE 1.
Microinjected pre-miR-31 is actively exported from nuclei of Xenopus laevis oocytes. Here, 15 nL of a mixture of 10 nM pre-miR-31 and U6Δss injection control were coinjected into nuclei of stage IV–V oocytes. If indicated, RNA mixtures also included 2 μM RanGAP or 500 nM or 2 μM unlabeled pre-miR-31 (competitor). Oocytes were separated into (N) nuclear and (C) cytoplasmic fractions 30 min after injection; RNA was extracted and analyzed on a 10% denaturing PAGE gel. The dried gel was quantified by a phoshorimager, and export was expressed as the cytoplasmic:nuclear (C:N) ratio.
FIGURE 2.
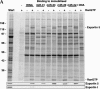
Exportin 5 forms export complexes with pre-miRNAs as well as with tRNA. (A) To identify a potential nuclear export receptor for pre-miRNAs, we first removed competing, endogenous RNAs from a HeLa cell lysate (see Materials and Methods) and bound the resulting extract (Start) to immobilized tRNA, indicated pre-miRNAs, or double-stranded DNA. Where indicated, 2 μM RanQ69L (GTP-form) had been included. Analysis was by SDS-PAGE, followed by Coomassie staining or immunoblotting with indicated antibodies. The load of bound material corresponds to 20 times the input. Note that Exp5 bound to tRNA and to any of the pre-miRNAs specifically and in an RanGTP-dependent manner. In contrast, exportin-t formed export complexes with tRNA only. None of the exportins bound immobilized dsDNA, which served as a control for nonspecific ionic interactions. (B) Escherichia coli lysate from bacteria expressing human Exp5 was depleted of endogenous RNA and subjected to binding to either immobilized tRNA or various pre-miRNAs in the absence or presence of RanQ69L GTP (2 μM). Binding assays were performed and analyzed as described in A.
FIGURE 2.
Exportin 5 forms export complexes with pre-miRNAs as well as with tRNA. (A) To identify a potential nuclear export receptor for pre-miRNAs, we first removed competing, endogenous RNAs from a HeLa cell lysate (see Materials and Methods) and bound the resulting extract (Start) to immobilized tRNA, indicated pre-miRNAs, or double-stranded DNA. Where indicated, 2 μM RanQ69L (GTP-form) had been included. Analysis was by SDS-PAGE, followed by Coomassie staining or immunoblotting with indicated antibodies. The load of bound material corresponds to 20 times the input. Note that Exp5 bound to tRNA and to any of the pre-miRNAs specifically and in an RanGTP-dependent manner. In contrast, exportin-t formed export complexes with tRNA only. None of the exportins bound immobilized dsDNA, which served as a control for nonspecific ionic interactions. (B) Escherichia coli lysate from bacteria expressing human Exp5 was depleted of endogenous RNA and subjected to binding to either immobilized tRNA or various pre-miRNAs in the absence or presence of RanQ69L GTP (2 μM). Binding assays were performed and analyzed as described in A.
FIGURE 3.
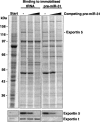
tRNA and pre-miRNA appear to bind to overlapping sites on Exp5. Binding of Exp5 from HeLa extract to immobilized tRNA and pre-miRNA was performed in the presence of RanQ69L (GTP) as described in Figure 2A ▶. Where indicated, 0.4 μM or 4 μM nonimmobilized pre-miR-31 had been added. The soluble pre-miRNA competed not only binding of Exp5 to immobilized pre-miRNA, but also to tRNA, which points to overlapping binding sites for tRNA and pre-miRNA on Exp5. The interaction between Exp-t and tRNA was not competed by pre-miRNA.
FIGURE 4.
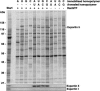
Exportin 5 binds double-stranded RNA in a sequence-independent manner. Immobilized RNA homopolymers (30 nt) were used as ssRNA or annealed with the complementary homopolymer (dsRNA) to retrieve interacting proteins from cytosolic HeLa extract depleted of endogenous RNA. HeLa extract was fractionated and binding assays were performed as described for Figure 2 ▶. Formation of export complexes was analyzed by SDS-PAGE, followed by Coomassie staining. Western blotting confirmed that Exp5 (but not Exp-t) could be retrieved with dsRNA independent of nucleotide composition and sequence, provided RanQ69L GTP had been present.
FIGURE 5.
Antibodies against exportin 5 block export of pre-miR-31 from nuclei of Xenopus oocytes. (A) Here, 15 nL of a mixture of radiolabeled 10 nM pre-miR-31 and U6Δss nuclear injection control were coinjected into nuclei of stage IV–V oocytes. Both RNA mixtures contained 1 μM unlabeled pre-miR-31, 1 Unit/μL Superasin (Ambion), and either 2 mg/mL anti-_Xenopus_-exportin-5 antibody (αExp5) or the same concentration of a control antibody (anti-_Drosophila_-exportin-5, which does not cross-react with the Xenopus protein). Oocytes were separated into (N) nuclear and (C) cytoplasmic fractions 30 min after injection; RNA was extracted and analyzed on a 10% denaturing PAGE gel. (B) In this panel, 15 nL of a mixture of radiolabeled 10 nM U6Δss injection control, tRNAPhe, and 7 nM U1ΔSm were coinjected into nuclei of stage IV–V oocytes. Injection mixtures contained 1 μM unlabeled tRNAPhe, 1 Unit/μL Superasin (Ambion), and either 2 mg/mL affinity-purified anti-_Xenopus_-exportin-5 or control antibodies as indicated. Oocytes were separated into nuclear and cytoplasmic fractions either immediately (0 min), 45, 90, or 180 min after injection and processed as in A. Each timepoint in both panels represents the average of five oocytes.
Similar articles
- Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs.
Yi R, Qin Y, Macara IG, Cullen BR. Yi R, et al. Genes Dev. 2003 Dec 15;17(24):3011-6. doi: 10.1101/gad.1158803. Epub 2003 Dec 17. Genes Dev. 2003. PMID: 14681208 Free PMC article. - Nuclear export of microRNA precursors.
Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Lund E, et al. Science. 2004 Jan 2;303(5654):95-8. doi: 10.1126/science.1090599. Epub 2003 Nov 20. Science. 2004. PMID: 14631048 - Exportin-5 mediates nuclear export of minihelix-containing RNAs.
Gwizdek C, Ossareh-Nazari B, Brownawell AM, Doglio A, Bertrand E, Macara IG, Dargemont C. Gwizdek C, et al. J Biol Chem. 2003 Feb 21;278(8):5505-8. doi: 10.1074/jbc.C200668200. Epub 2002 Dec 30. J Biol Chem. 2003. PMID: 12509441 - MicroRNA precursors in motion: exportin-5 mediates their nuclear export.
Kim VN. Kim VN. Trends Cell Biol. 2004 Apr;14(4):156-9. doi: 10.1016/j.tcb.2004.02.006. Trends Cell Biol. 2004. PMID: 15134074 Review. - Ran-dependent nuclear export mediators: a structural perspective.
Güttler T, Görlich D. Güttler T, et al. EMBO J. 2011 Aug 31;30(17):3457-74. doi: 10.1038/emboj.2011.287. EMBO J. 2011. PMID: 21878989 Free PMC article. Review.
Cited by
- Role of microRNAs in the immune system, inflammation and cancer.
Raisch J, Darfeuille-Michaud A, Nguyen HT. Raisch J, et al. World J Gastroenterol. 2013 May 28;19(20):2985-96. doi: 10.3748/wjg.v19.i20.2985. World J Gastroenterol. 2013. PMID: 23716978 Free PMC article. Review. - Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells.
Mace TA, Collins AL, Wojcik SE, Croce CM, Lesinski GB, Bloomston M. Mace TA, et al. J Surg Res. 2013 Oct;184(2):855-60. doi: 10.1016/j.jss.2013.04.061. Epub 2013 May 18. J Surg Res. 2013. PMID: 23726431 Free PMC article. - Regulatory Non-Coding RNAs during Porcine Viral Infections: Potential Targets for Antiviral Therapy.
Li F, Yu H, Qi A, Zhang T, Huo Y, Tu Q, Qi C, Wu H, Wang X, Zhou J, Hu L, Ouyang H, Pang D, Xie Z. Li F, et al. Viruses. 2024 Jan 13;16(1):118. doi: 10.3390/v16010118. Viruses. 2024. PMID: 38257818 Free PMC article. Review. - The role of microRNAs in skeletal muscle health and disease.
Kirby TJ, Chaillou T, McCarthy JJ. Kirby TJ, et al. Front Biosci (Landmark Ed). 2015 Jan 1;20(1):37-77. doi: 10.2741/4298. Front Biosci (Landmark Ed). 2015. PMID: 25553440 Free PMC article. Review. - TRIM29 inhibits miR-873-5P biogenesis via CYTOR to upregulate fibronectin 1 and promotes invasion of papillary thyroid cancer cells.
Wu T, Zhang DL, Wang JM, Jiang JY, Du X, Zeng XY, Du ZX. Wu T, et al. Cell Death Dis. 2020 Sep 29;11(9):813. doi: 10.1038/s41419-020-03018-3. Cell Death Dis. 2020. PMID: 32994394 Free PMC article.
References
- Arts, G.J., Fornerod, M., and Mattaj, I.W. 1998a. Identification of a nuclear export receptor for tRNA. Curr. Biol. 8: 305–314. - PubMed
- Bollman, K.M., Aukerman, M.J., Park, M.Y., Hunter, C., Berardini, T.Z., and Poethig, R.S. 2003. HASTY, the Arabidopsis ortholog of exportin 5/MSN5, regulates phase change and morphogenesis. Development 130: 1493–1504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous